


Pharmaceutical companies are pivotal organizations committed to the research, development, manufacturing, and promotion of medications. They play a crucial role in healthcare, driving medical innovation and enhancing patient outcomes. This article underscores their substantial investment in research and development, the complexities of regulatory compliance, and their profound impact on public health and the economy. It illustrates how these companies are not only advancing medical science but also contributing significantly to economic growth.
Pharmaceutical companies stand at the forefront of healthcare innovation, driving advancements that shape the medical landscape. These organizations are not merely businesses; they are vital players in the research, development, and distribution of life-saving medications that address a multitude of health challenges.
As they navigate the complexities of regulatory environments and the high costs of drug development, a critical question emerges: how do these companies balance the pursuit of profit with the imperative to ensure public health and access to essential treatments?
Understanding this dynamic reveals the profound impact pharmaceutical companies have on both individual lives and the global economy.
A healthcare firm is an example of what is a pharmaceutical company, as it represents a commercial organization dedicated to the research, development, manufacturing, and promotion of medicines and treatments. These firms play a pivotal role in the healthcare system, delivering essential therapeutic products that address a diverse range of medical conditions. Their defining characteristics encompass a robust emphasis on innovation, unwavering adherence to regulatory standards, and a steadfast commitment to improving patient outcomes.
In 2023, drug manufacturers allocated approximately 21.4% of their total revenues to research and development (R&D), underscoring their dedication to discovering new medications—a process that often spans years and necessitates substantial financial investment. Furthermore, navigating intricate regulatory environments is imperative for these organizations to ensure that their products are both safe and effective for public use.
As of early 2025, more than 6,800 drug manufacturers are actively engaged in research and development, underscoring the sector's resolute commitment to medical innovation. Successful drug manufacturers not only drive advancements in treatment but also significantly influence healthcare policies and practices, ultimately enhancing the quality of care available to patients worldwide.

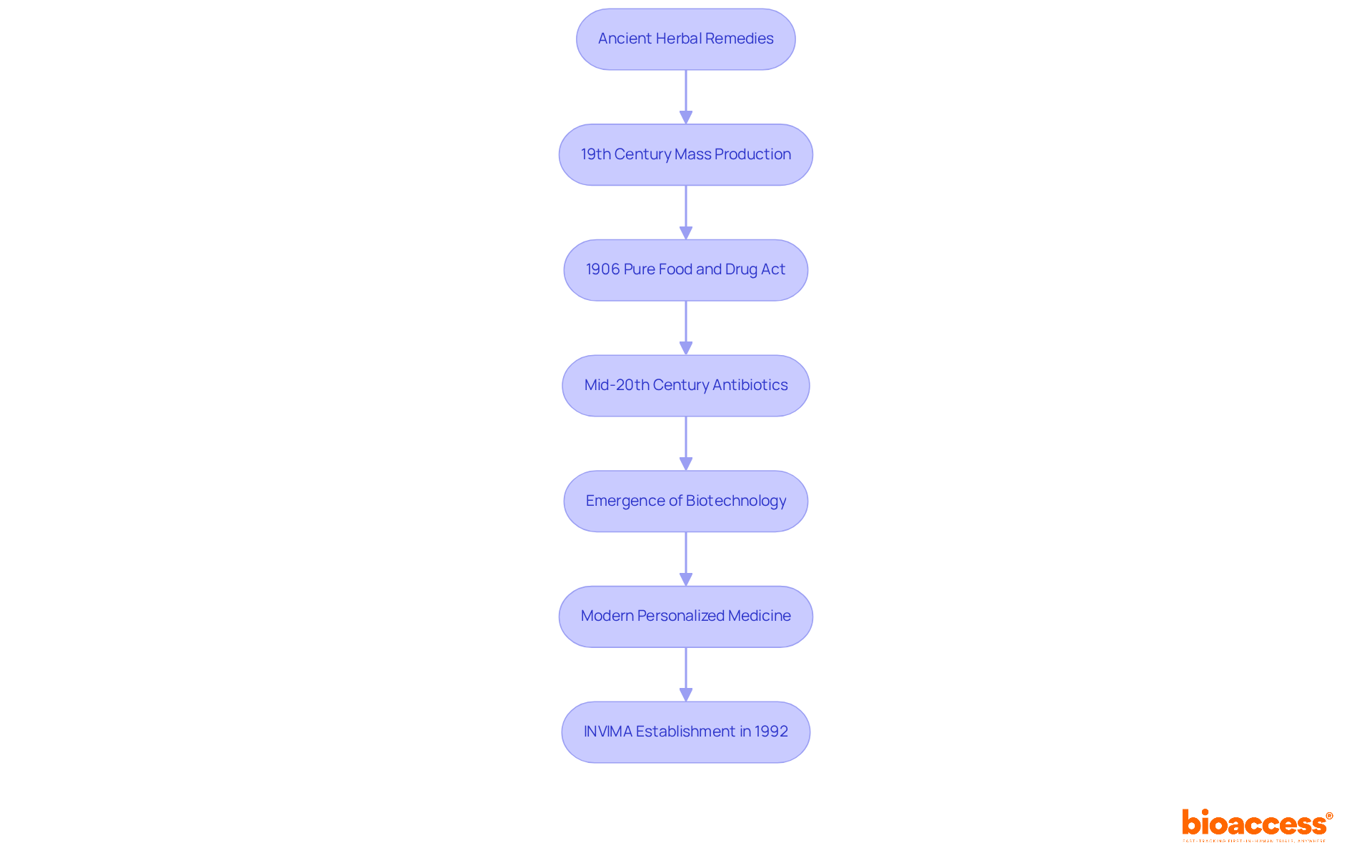
The medicine sector has its roots in ancient herbal remedies and apothecaries, evolving considerably in the 19th century with the founding of businesses focused on the mass production of medications. A pivotal milestone was the introduction of the Pure Food and Drug Act in 1906, which sought to regulate drug quality and safety. The mid-20th century marked another transformative period with the advent of antibiotics, fundamentally changing medical treatment. As the industry progressed, biotechnology firms emerged, driving a shift towards personalized medicine that leverages advancements in science and technology.
Today, understanding what is a pharmaceutical company involves recognizing how they lead in innovation by developing complex biologics and gene therapies that target previously untreatable conditions, reflecting a commitment to addressing diverse health challenges. In Colombia, the regulatory framework is significantly influenced by INVIMA (Colombia National Food and Drug Surveillance Institute), which oversees the inspection and supervision of health products. Founded in 1992, INVIMA guarantees adherence to health standards and optimal practices, serving an essential function in the approval process for medicines and medical devices. Its Directorate for Medical Devices monitors and controls medical devices, tracks pre- and post-market programs, and suggests technical standards for manufacturing and quality assurance.
INVIMA's classification as a Level 4 health authority by the Pan American Health Organization/World Health Organization underscores its competence in guaranteeing the safety, efficacy, and quality of medicines. This regulatory oversight promotes confidence in pharmaceutical products and aids the local economy by enabling clinical studies and global partnerships that stimulate job creation and advancements in medical services.
To understand what is a pharmaceutical company, it's important to recognize their essential role in medical innovation, managing the complete drug development lifecycle from discovery to market introduction. They conduct extensive clinical trials to evaluate the safety and effectiveness of new medications, frequently collaborating with academic institutions and medical providers to enhance research outcomes. As of 2025, the cooperation between pharmaceutical firms and medical providers is increasingly recognized as vital for fostering innovation and improving patient care.
What is a pharmaceutical company? These companies are responsible for ensuring compliance with stringent regulatory requirements, necessitating meticulous documentation and reporting. Navigating the regulatory approval process is critical for the successful launch of new therapies. Their commitment to research and development not only leads to the introduction of new therapies but also exemplifies what is a pharmaceutical company that stimulates economic growth and job creation within the healthcare sector. For instance, the worldwide market for contract research outsourcing was estimated at around $49.8 billion in 2022, with forecasts suggesting it might reach $90.4 billion by 2030. This highlights what is a pharmaceutical company’s growing reliance on cooperative initiatives in the development of pharmaceuticals.
Furthermore, drug manufacturers engage in post-marketing surveillance to monitor the long-term effects of their products, ensuring continuous safety and effectiveness. A notable statistic reveals that only approximately 10% of medications that enter clinical trials ultimately secure FDA approval, underscoring the necessity of effective collaboration and robust clinical trial designs.
Case studies illustrate the impact of these collaborations. For example, partnerships between pharmaceutical firms and academic institutions have led to innovative trial designs that enhance patient recruitment and retention, addressing the challenge that roughly 80% of clinical trials fail to meet initial enrollment targets. This shortfall can result in significant financial losses, estimated at $8 million in revenue per day for drug discovery companies. Insights from clinical research leaders emphasize that nurturing strong connections with providers is essential for promoting medical innovation, which is a key aspect of what is a pharmaceutical company, and ensuring that new treatments adequately meet patient needs. Additionally, bioaccess® exemplifies the economic impact of these collaborations by offering comprehensive clinical trial management services, contributing to local economies through job creation and improved healthcare outcomes.
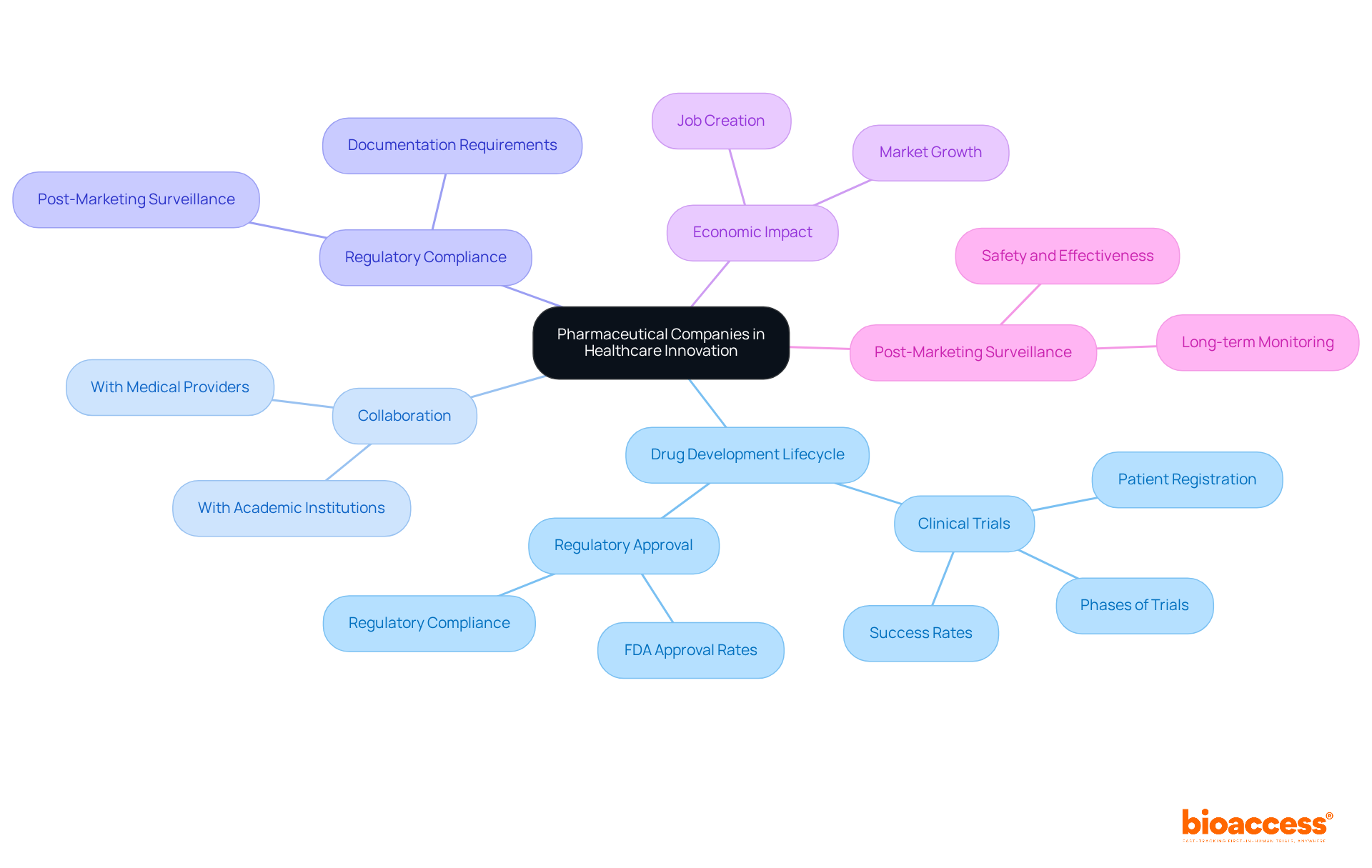
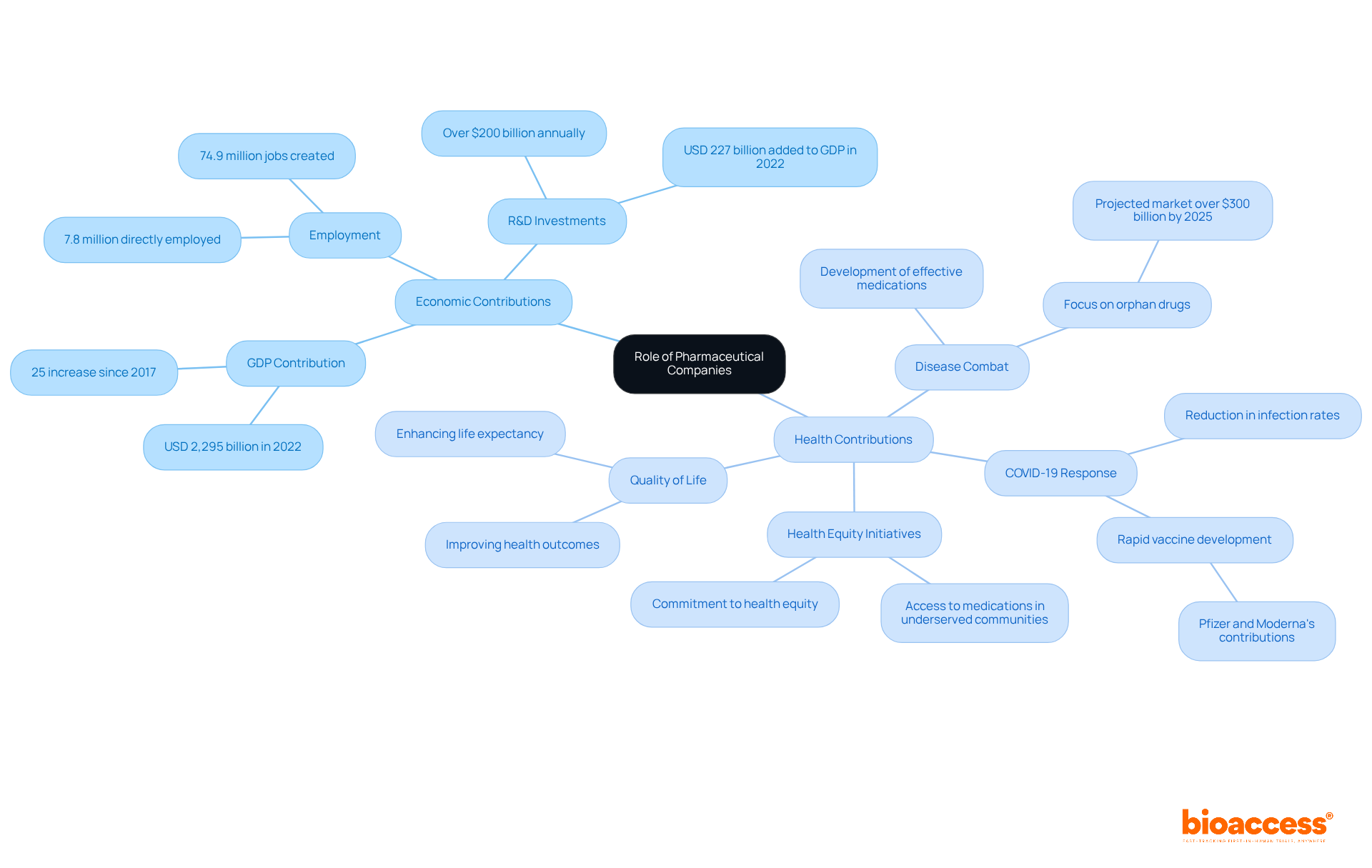
To understand what is a pharmaceutical company, it's important to recognize that these firms play a pivotal role in the global economy, generating trillions in revenue and employing millions worldwide. In 2022, the industry contributed approximately USD 2,295 billion to global GDP, with direct employment of 7.8 million people. Their substantial investments in research and development, exceeding $200 billion annually, not only foster innovative treatments but also stimulate economic activity through job creation and infrastructure development.
Health-wise, what is a pharmaceutical company is vital for combating diseases, enhancing quality of life, and increasing life expectancy through the development of effective medications. The COVID-19 pandemic underscored their significance in public health, as companies like Pfizer and Moderna rapidly developed vaccines, leading to a notable reduction in infection rates and hospitalizations.
Furthermore, drug companies often engage in initiatives aimed at enhancing access to medications in underserved communities, demonstrating their commitment to health equity. For instance, the orphan drug market, projected to exceed $300 billion by 2025, highlights the industry's focus on addressing rare diseases, ensuring that even niche health needs are met.
Overall, the contributions of pharmaceutical firms extend beyond mere profit; they are integral to advancing public health and fostering economic resilience.
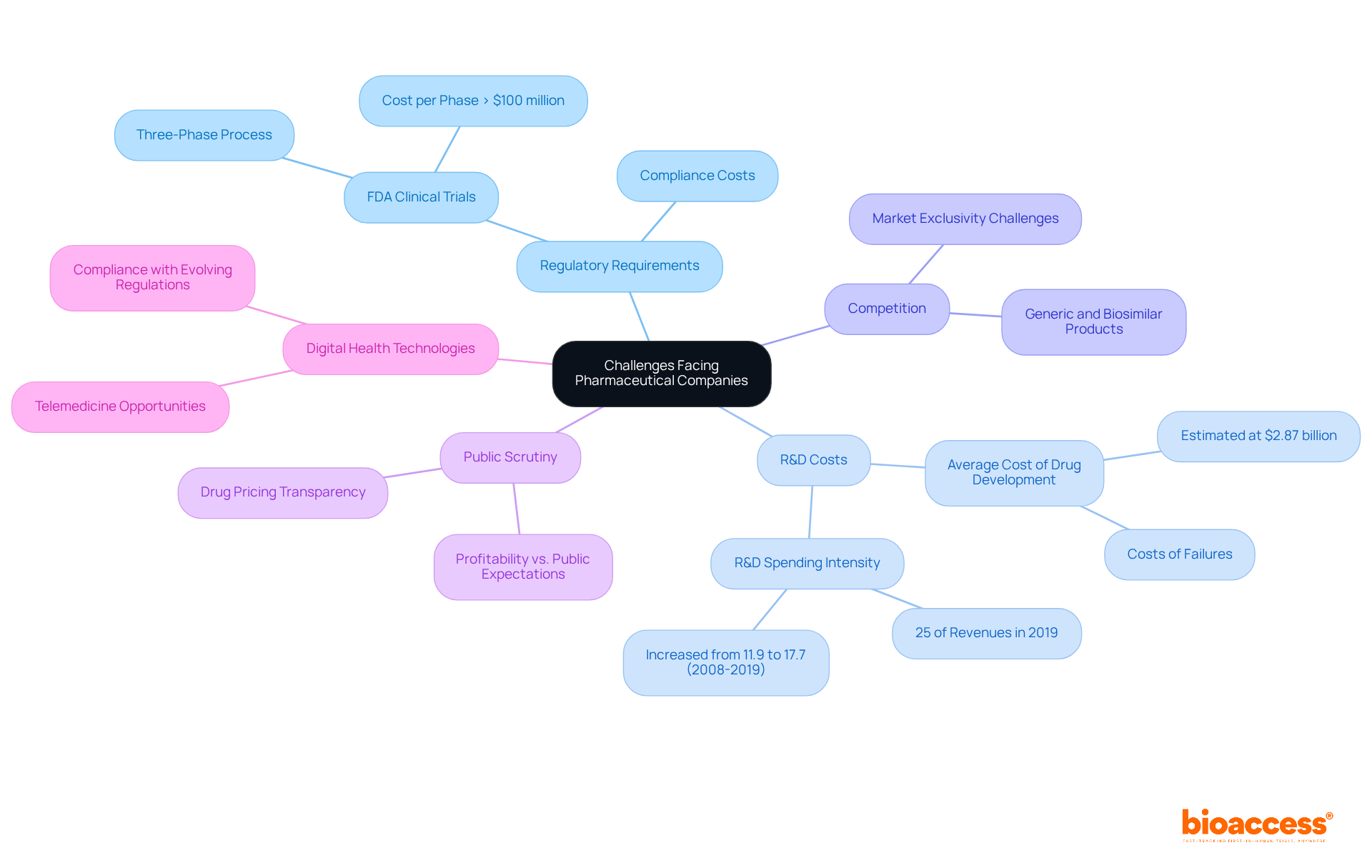
In the context of today's landscape, what is a pharmaceutical company? It is an organization that faces numerous challenges, primarily driven by stringent regulatory requirements, escalating research and development (R&D) costs, and increasing competition from generic and biosimilar products. The medication development process is notoriously lengthy and costly, often spanning more than ten years and requiring upwards of $2 billion, which can deter investment and stifle innovation. For instance, the Tufts Center for the Study of Pharmaceutical Development estimated that the average cost of creating a new medication reached $2.87 billion in 2014, a figure that has faced scrutiny regarding its transparency and reliability.
Public scrutiny surrounding drug pricing and access has intensified, prompting enterprises to adopt more transparent pricing strategies. This shift is crucial, as the profitability of large drug firms has significantly exceeded that of non-drug enterprises, with operating profits climbing from 15.3% of sales in 1979 to 23.4% in 2018. Such financial dynamics underscore the imperative for drug manufacturers to strike a balance between profitability and public expectations.
Furthermore, the emergence of digital health technologies and telemedicine introduces both opportunities and challenges. Companies must adapt to innovative medical delivery methods while ensuring compliance with evolving regulatory frameworks. The FDA's rigorous three-phase clinical trial process, which can incur costs exceeding $100 million per phase, exemplifies the regulatory hurdles that can delay timely market entry.
To navigate these complexities, it is essential to understand what is a pharmaceutical company and how they are increasingly prioritizing collaboration with various stakeholders, including governments, healthcare providers, and patient advocacy groups. This collaborative approach seeks to foster innovation and enhance patient access to medications, ultimately addressing the pressing challenges of regulatory compliance and market dynamics.
Pharmaceutical companies serve as the backbone of the healthcare system, dedicated to the research, development, and distribution of essential medications that improve patient outcomes and address a myriad of health challenges. Their unwavering commitment to innovation, regulatory compliance, and economic growth reflects their vital role in advancing public health and shaping healthcare practices globally.
The multifaceted nature of pharmaceutical companies is highlighted through their historical evolution and significant economic contributions, as well as their responsibilities in healthcare innovation. With substantial investments in research and development, these companies not only create groundbreaking therapies but also navigate complex regulatory landscapes to ensure the safety and efficacy of their products. The collaboration between pharmaceutical firms and various stakeholders, including healthcare providers and regulatory bodies, is crucial for fostering innovation and enhancing patient access to medications.
Ultimately, the significance of pharmaceutical companies extends beyond profit margins; they are integral to the advancement of public health, economic resilience, and the continuous evolution of medical treatments. As the industry faces challenges such as rising R&D costs and regulatory pressures, a collective effort among stakeholders is essential to ensure that the benefits of pharmaceutical innovations reach all corners of society. Engaging with these companies and advocating for transparency and accessibility can help pave the way for a healthier future for everyone.
What defines a pharmaceutical company?
A pharmaceutical company is a commercial organization dedicated to the research, development, manufacturing, and promotion of medicines and treatments, playing a crucial role in the healthcare system by delivering therapeutic products for various medical conditions.
What are the core characteristics of pharmaceutical companies?
Core characteristics of pharmaceutical companies include a strong emphasis on innovation, strict adherence to regulatory standards, and a commitment to improving patient outcomes.
How much do drug manufacturers invest in research and development?
In 2023, drug manufacturers allocated approximately 21.4% of their total revenues to research and development (R&D), highlighting their dedication to discovering new medications.
How many drug manufacturers are involved in research and development as of early 2025?
As of early 2025, more than 6,800 drug manufacturers are actively engaged in research and development.
What historical milestones have shaped the pharmaceutical industry?
Key milestones include the introduction of the Pure Food and Drug Act in 1906, the advent of antibiotics in the mid-20th century, and the emergence of biotechnology firms that have driven advancements in personalized medicine.
What role does INVIMA play in the pharmaceutical industry in Colombia?
INVIMA (Colombia National Food and Drug Surveillance Institute) oversees the inspection and supervision of health products, ensuring adherence to health standards and optimal practices, thus playing a critical role in the approval process for medicines and medical devices.
What is INVIMA's classification and its significance?
INVIMA is classified as a Level 4 health authority by the Pan American Health Organization/World Health Organization, underscoring its competence in ensuring the safety, efficacy, and quality of medicines, which promotes confidence in pharmaceutical products and supports the local economy.
How do pharmaceutical companies influence healthcare policies?
Successful drug manufacturers drive advancements in treatment and significantly influence healthcare policies and practices, ultimately enhancing the quality of care available to patients worldwide.