


Analytical methods serve as a systematic process essential for identifying the composition and characteristics of substances. Techniques such as chromatography and mass spectrometry play a pivotal role in medical research, particularly in evaluating the safety and effectiveness of healthcare products. These methods are not just technicalities; they are crucial for ensuring accurate data collection and regulatory compliance. This, in turn, contributes significantly to successful clinical trials and improved patient outcomes.
In the ever-evolving Medtech landscape, the importance of these analytical techniques cannot be overstated. They address key challenges faced in clinical research, ensuring that healthcare products meet stringent safety standards. By employing these methods, researchers can gather reliable data that supports their findings, fostering trust in the healthcare system.
Ultimately, collaboration among researchers, regulatory bodies, and healthcare providers is vital. As we move forward, embracing these analytical methods will be essential in navigating the complexities of clinical trials and enhancing patient care. The next steps involve not only adopting these techniques but also fostering partnerships that prioritize safety and efficacy in healthcare.
Understanding the complexities of analytical methods is essential in scientific research, especially in the medical field, where precision can significantly influence patient outcomes. These systematic approaches not only help identify the composition and characteristics of substances but also play a crucial role in developing and validating healthcare products. As the landscape of clinical trials evolves, researchers encounter the urgent challenge of ensuring their methods remain compliant and effective amid stringent regulatory standards.
What are the key components that define an analytical method? How can researchers navigate these complexities to enhance their efficacy and reliability? These questions are vital as they guide the exploration of analytical methods in clinical research, emphasizing the need for a robust understanding of the tools at their disposal.
A systematic approach is essential in understanding what is analytical method, as it refers to a structured process used to identify the composition, structure, or characteristics of a substance through various techniques and procedures. In medical research, assessment techniques are vital for determining the effectiveness and safety of healthcare products, including pharmaceuticals and medical devices. Chromatography, mass spectrometry, and spectrophotometry are examples of what is analytical method, as they provide essential data that inform regulatory decisions and treatment outcomes.
To effectively support the development and validation of these evaluation techniques, comprehensive clinical trial management services are indispensable. These services include:
Additionally, obtaining necessary import licenses and overseeing the entire project are crucial to ensure that evaluation techniques meet the required quality and safety criteria, ultimately influencing patient health and treatment effectiveness.
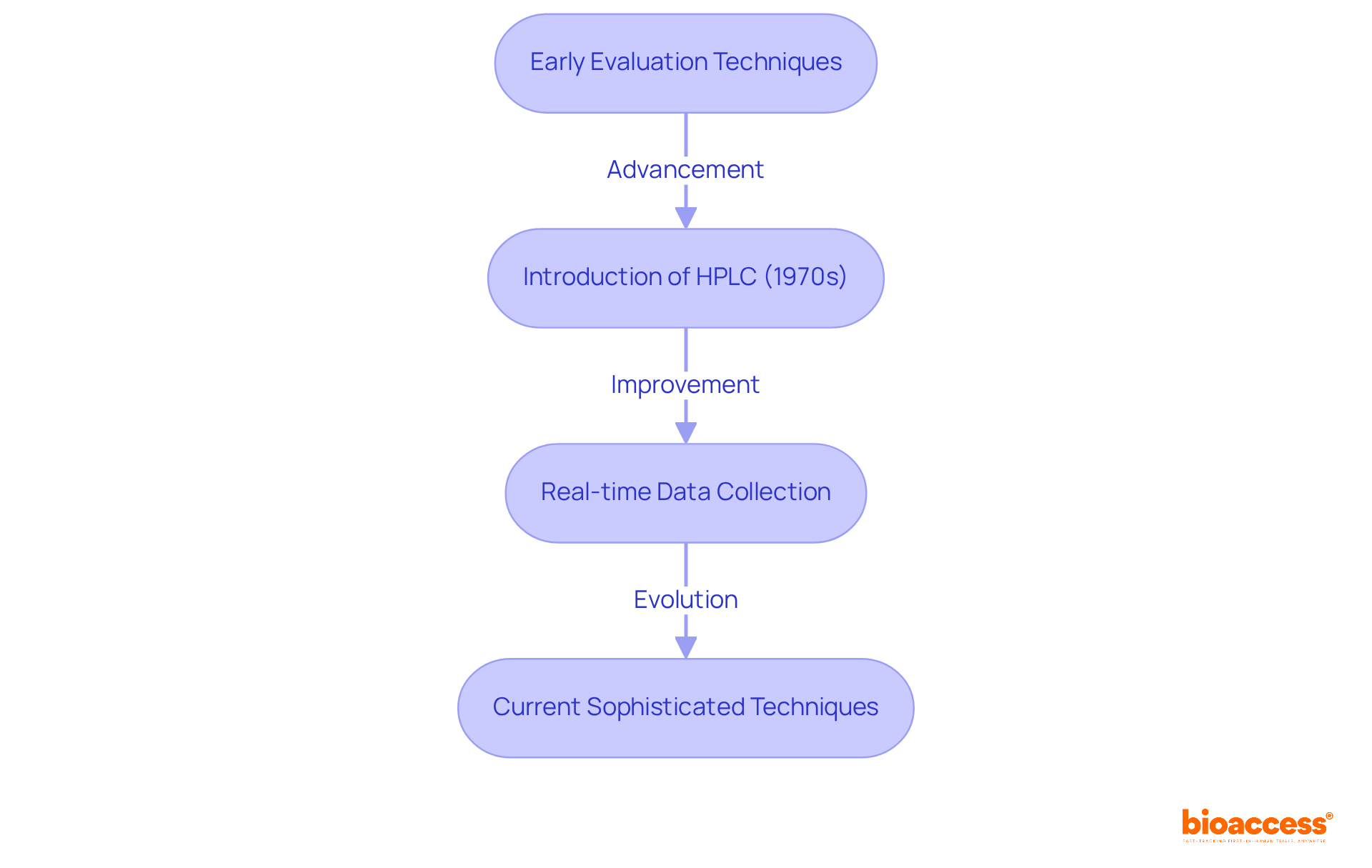
The evolution of evaluation techniques in medical research has its roots in the early days of medicine, where basic strategies were employed to assess the characteristics of various substances. Over the years, advancements in technology and scientific understanding have led to the development of sophisticated techniques. A prime example is the introduction of high-performance liquid chromatography (HPLC) in the 1970s, which transformed how researchers separate and analyze compounds. Today, evaluation techniques are not only more accurate but also more effective, allowing for real-time data collection and analysis—an essential requirement in the fast-paced environment of medical trials.
This ongoing evolution underscores the critical need for accuracy and reliability in research, ensuring that new therapies are both safe and effective. Furthermore, bioaccess enhances the efficacy of these analytical methods, which raises the question of what is analytical method, through its comprehensive clinical trial management services. These services include:
By ensuring rigorous oversight and adherence to regulatory standards, bioaccess facilitates successful trials and contributes to local economies through job creation and healthcare improvements, fostering international collaboration within the medtech sector.
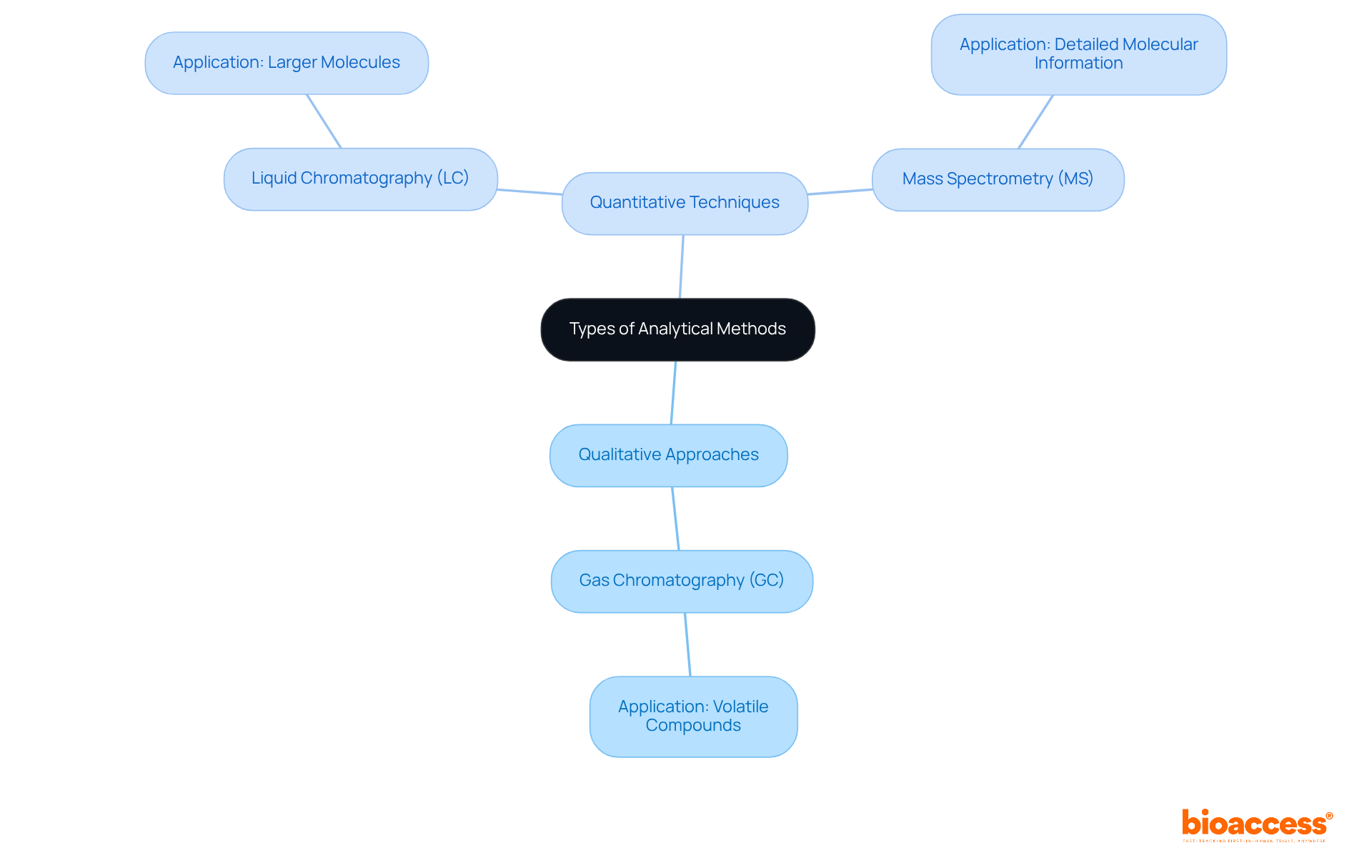
In clinical research, understanding what is analytical method is crucial, as these approaches can be classified into several types, each with distinct traits and applications. For instance, qualitative approaches are essential for determining the presence of a substance, while quantitative techniques focus on assessing the quantity of that substance. Techniques such as gas chromatography (GC) are commonly employed for volatile compounds, whereas liquid chromatography (LC) is preferred for larger molecules. Additionally, mass spectrometry (MS) often complements these techniques, providing detailed molecular information that is vital in medical studies.
These analytical techniques are indispensable in medication development, quality assurance, and regulatory adherence, ensuring that products are safe for human consumption. Understanding what is analytical method along with the strengths and limitations of each approach empowers researchers to select the most suitable technique for their specific study requirements. By leveraging these methods effectively, researchers can navigate the complexities of the Medtech landscape and address key challenges in clinical research.
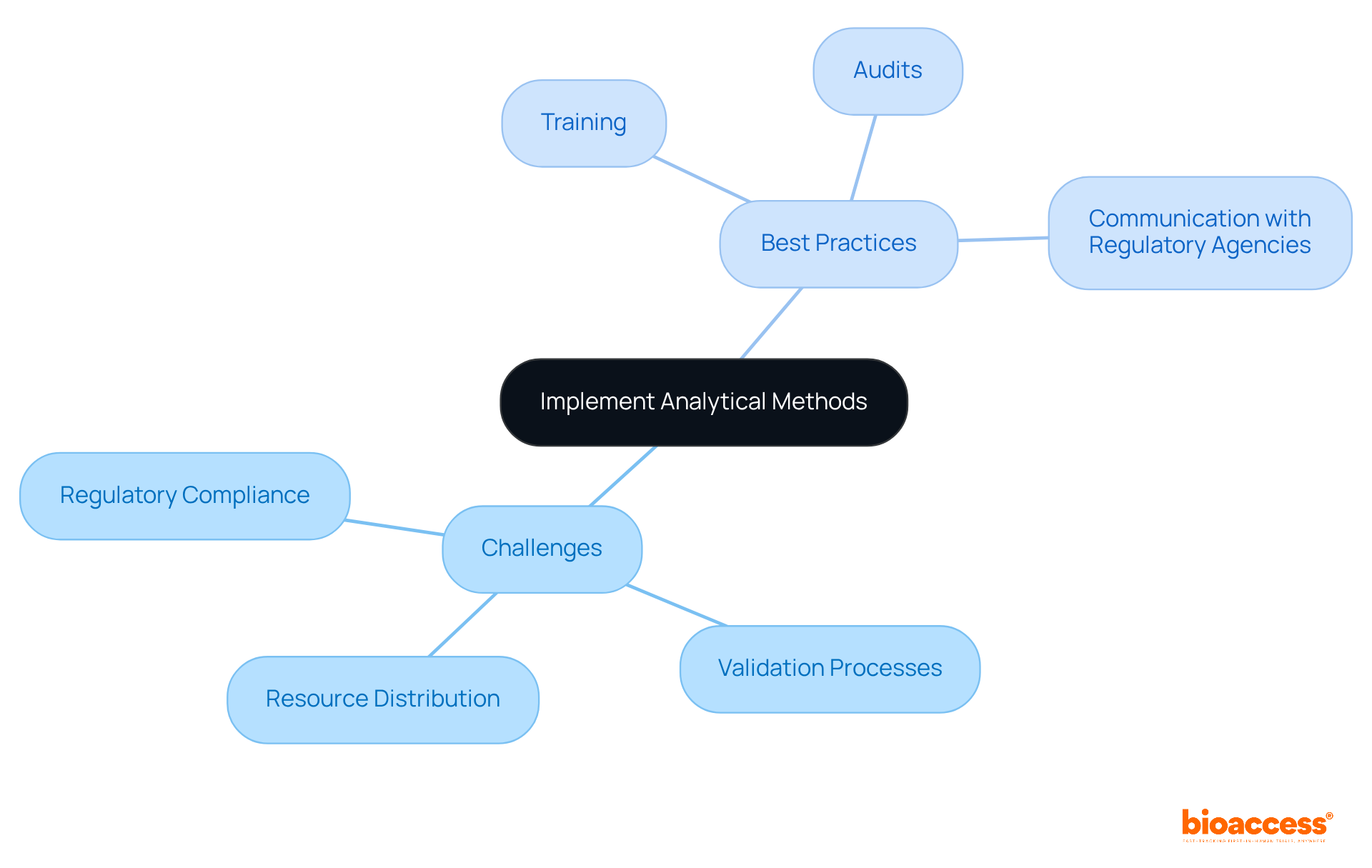
Applying evaluative techniques in clinical research presents a unique set of challenges, including regulatory compliance, validation processes, and resource distribution. Ensuring that assessment techniques meet regulatory requirements is not just important; it’s essential. Any inconsistencies can lead to significant delays in product authorization, which can be detrimental to research timelines. Moreover, validating these techniques is crucial to confirm that the chosen approach is suitable for its intended purpose.
To effectively tackle these challenges, comprehensive training for research personnel is vital. Regular audits of evaluation processes help maintain high standards, while open communication with regulatory agencies fosters a collaborative environment. By adhering to these practices, researchers can significantly enhance the reliability and efficiency of what is analytical method. This ultimately leads to improved clinical outcomes, reinforcing the importance of diligence in the research process.
In the ever-evolving Medtech landscape, the role of bioaccess in addressing these key challenges cannot be overstated. As researchers navigate the complexities of clinical trials, they must consider how these evaluative techniques can be optimized. What steps can you take to ensure your assessment methods are both compliant and effective? By prioritizing these strategies, you position yourself for success in clinical research.
Understanding analytical methods is crucial in clinical research, as these structured processes are vital for identifying the composition and characteristics of substances. Techniques like chromatography, mass spectrometry, and spectrophotometry empower researchers to ensure the safety and effectiveness of healthcare products, ultimately impacting patient outcomes and regulatory decisions.
This article delves into the evolution and application of analytical methods. The historical development showcases significant advancements, such as high-performance liquid chromatography, which transformed compound analysis. Additionally, classifying analytical methods into qualitative and quantitative approaches highlights their diverse applications in medication development and regulatory compliance. Challenges in implementing these methods, including regulatory adherence and validation, underscore the necessity for comprehensive training and open communication with regulatory bodies.
In summary, the importance of analytical methods in clinical research is undeniable. As the Medtech landscape evolves, researchers must prioritize optimizing these techniques to navigate the complexities of clinical trials effectively. By adopting best practices and remaining vigilant in compliance and validation, the medical community can enhance research outcomes and contribute to the development of safe and effective therapies. The call to action is clear: invest in robust analytical methods to ensure success in the competitive field of clinical research.
What is an analytical method?
An analytical method is a systematic process used to identify the composition, structure, or characteristics of a substance through various techniques and procedures.
Why are analytical methods important in medical research?
Analytical methods are vital for determining the effectiveness and safety of healthcare products, including pharmaceuticals and medical devices.
What are some examples of analytical methods?
Examples of analytical methods include chromatography, mass spectrometry, and spectrophotometry.
How do analytical methods impact regulatory decisions and treatment outcomes?
Analytical methods provide essential data that inform regulatory decisions and influence treatment outcomes, ensuring the safety and efficacy of healthcare products.
What services support the development and validation of evaluation techniques?
Comprehensive clinical trial management services support this development, including feasibility studies, careful selection of principal investigators, compliance reviews, and efficient trial setup processes.
What is the significance of obtaining import licenses in analytical methods?
Obtaining necessary import licenses is crucial to ensure that evaluation techniques meet the required quality and safety criteria, which ultimately influences patient health and treatment effectiveness.