


Understanding clinical development is crucial for grasping how new medical interventions evolve from theoretical concepts into real-world applications. This intricate process not only determines the safety and efficacy of drugs and therapies but also plays a pivotal role in enhancing patient care and outcomes. However, with only a fraction of experimental drugs achieving regulatory approval and numerous studies facing delays, what challenges threaten the success of clinical trials? Exploring the phases, key players, and ethical considerations in clinical development reveals a complex landscape that is both critical and fraught with obstacles.
What is clinical development is a systematic process that involves researching and testing new medical interventions, such as drugs, devices, and therapies, to assess their safety and efficacy for human use. This phase is crucial in the healthcare sector, acting as a vital link between laboratory research and practical application. The primary goal of medical development is to produce reliable data that supports regulatory approval and informs practice, ultimately enhancing patient outcomes.
The medical development process is organized into multiple phases, each designed to address specific inquiries about the intervention's impact on health and illness. For instance, statistics reveal that only 10% of experimental drugs receive FDA approval, highlighting the significant challenges faced in this arena. Additionally, around 80% of research studies encounter delays or shutdowns due to recruitment challenges, underscoring the urgent need for effective strategies in patient involvement.
Real-world examples illustrate what clinical development can do to successfully bridge laboratory research and clinical application. The incorporation of real-world evidence (RWE) has shown an increase in patient recruitment and retention by up to 20%, providing insights into patient behavior that enhance study designs. Moreover, the implementation of decentralized studies, accelerated by the COVID-19 pandemic, has proven viable and advantageous, allowing for quicker enrollment and increased diversity among participants. This shift not only boosts recruitment success but also helps to clarify what is clinical development by aligning studies more closely with real-world patient experiences, ultimately leading to more effective healthcare solutions.
In this context, bioaccess® stands out by enabling treatment-naive cardiology or neurology cohorts to enroll 50% faster than Western sites, achieving significant cost savings of $25K per patient with FDA-ready data-no rework, no delays. Partnerships, such as the one with Welwaze Medical Inc. for the Celbrea® medical device introduction in Colombia, demonstrate how bioaccess™ helps overcome regulatory and market access obstacles, further enhancing the effectiveness of studies. Furthermore, the pharmaceutical sector could save up to $50 billion each year by integrating RWE into clinical studies, emphasizing the financial impact of these strategies on clinical advancement.
Clinical development is systematically divided into several key phases, each serving a distinct purpose in the drug development process:
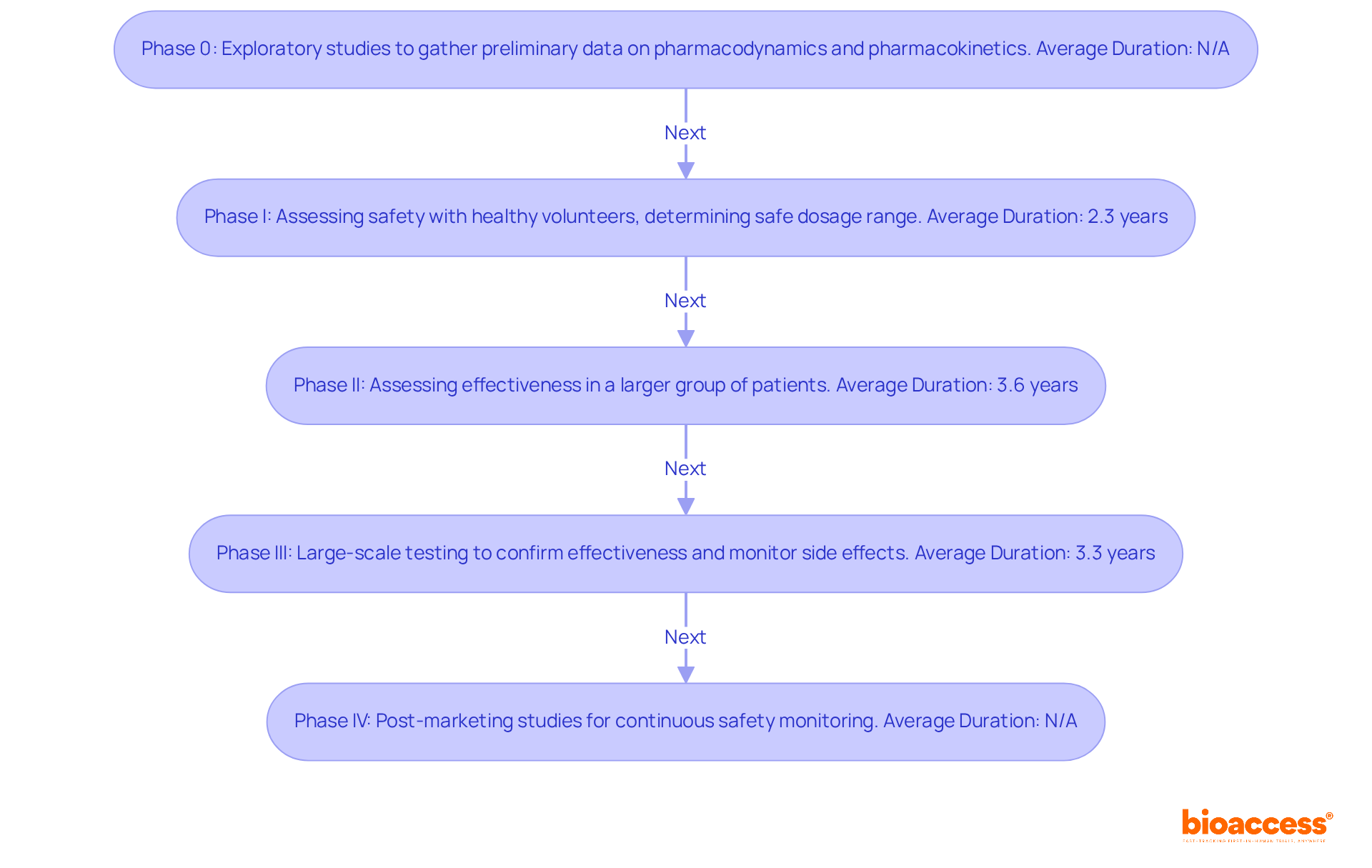
Phase 0: These exploratory studies involve a small number of subjects and aim to gather preliminary data on pharmacodynamics and pharmacokinetics, setting the stage for further research.
Phase I: This phase is crucial for assessing safety. It typically involves a small group of healthy volunteers and focuses on determining the safe dosage range while identifying potential side effects. The average duration for Phase I trials is approximately 2.3 years, but CNS trials may take only about 11.2 months. Despite thorough testing, only around 7.9% of compounds in Phase I development ultimately obtain approval, emphasizing the difficulties inherent in this stage.
Phase II: In this phase, the effectiveness of the medication or treatment is assessed in a larger group of patients who have the condition the medication is meant to address. This phase continues to evaluate safety while offering essential information on the treatment's effectiveness, with an average duration of about 3.6 years.
Phase III: This phase involves large-scale testing to confirm effectiveness, monitor side effects, and compare the new intervention to standard or equivalent treatments. The average duration for Phase III trials has increased to approximately 3.3 years, reflecting the growing complexity of these studies. The typical duration between Phase III and regulatory approval is approximately 1.3 years, highlighting the extended procedure before a treatment reaches the market.
Phase IV: These post-marketing studies collect further information on the medication's risks, benefits, and optimal use after it has been approved for public use. This phase is essential for continuous safety monitoring and can lead to new insights about the medication's long-term effects.
To understand what is clinical development, it is important to recognize that each phase is critical in ensuring that only safe and effective treatments reach the market. As the industry progresses, innovations like big data and AI are being utilized to enhance research processes, possibly decreasing the time and expenses related to drug development. bioaccess® employs these technologies to improve their management services for studies, ensuring effective navigation through the intricacies of the Latin American market.
In this context, bioaccess® provides extensive research management services, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies (PMCF). Their proficiency in overseeing these stages guarantees that innovative Medtech, Biopharma, and Radiopharma startups can expedite their trials and obtain regulatory approval more effectively. The importance of these phases cannot be overstated, as they collectively contribute to the rigorous standards required for bringing new therapies to patients.
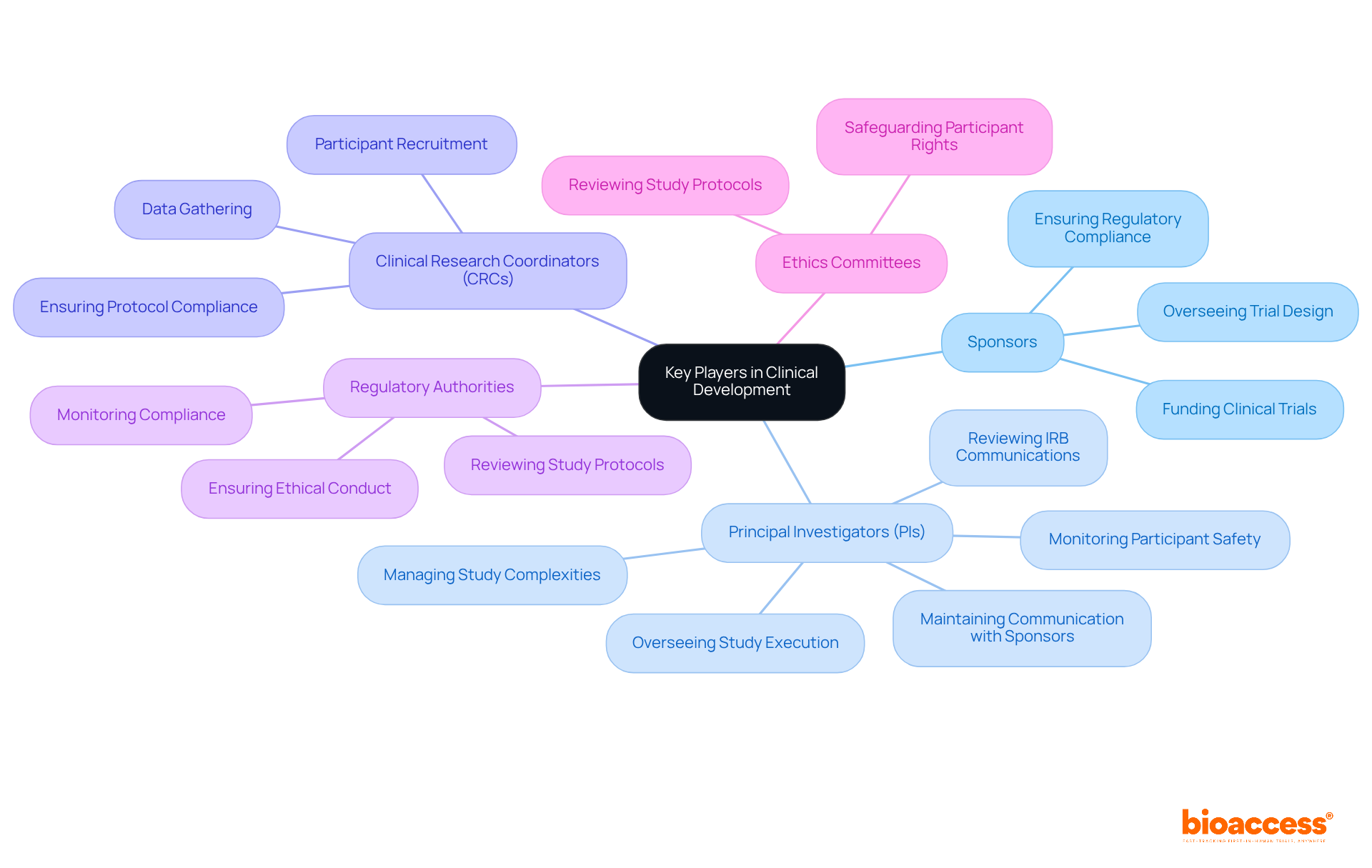
The clinical development process is a collaborative effort involving several key stakeholders, each with distinct responsibilities that are vital for the successful execution of clinical trials:
Sponsors: Primarily pharmaceutical and biotechnology companies, sponsors are responsible for funding and overseeing clinical trials. They play a crucial role in ensuring that tests are designed to meet regulatory standards and scientific objectives.
Principal Investigators (PIs): PIs oversee the overall execution of studies at their locations. They ensure compliance with regulatory requirements and ethical standards, actively monitor participant safety, and maintain communication with sponsors and regulatory authorities. Their responsibilities include reviewing and certifying all IRB communications and ensuring that all essential documents are up-to-date and audit-ready. Recent trends indicate that PIs must also manage growing complexities in studies, with the average number of eligibility criteria per study increasing significantly in recent years.
Clinical Research Coordinators (CRCs): Collaborating closely with PIs, CRCs oversee the daily activities of research studies. Their responsibilities consist of participant recruitment, data gathering, and ensuring compliance with study protocols, which is crucial for maintaining the integrity of the research.
Regulatory Authorities: Organizations such as the FDA and EMA are responsible for reviewing and approving clinical study protocols. They monitor compliance with regulations to ensure that trials are conducted safely and ethically, reflecting the growing emphasis on regulatory responsiveness in the industry.
Ethics Committees: These committees review study protocols to safeguard participants' rights and welfare. Their oversight is crucial in maintaining ethical standards throughout the medical development process.
Each of these roles is essential to the successful implementation of medical studies, ensuring that research is carried out ethically and efficiently. As the trial landscape evolves, collaboration among these stakeholders becomes increasingly important. Notably, the global market for contract research outsourcing is projected to reach $90.4 billion by 2030, highlighting the growing reliance on CROs and the need for effective management of trials.
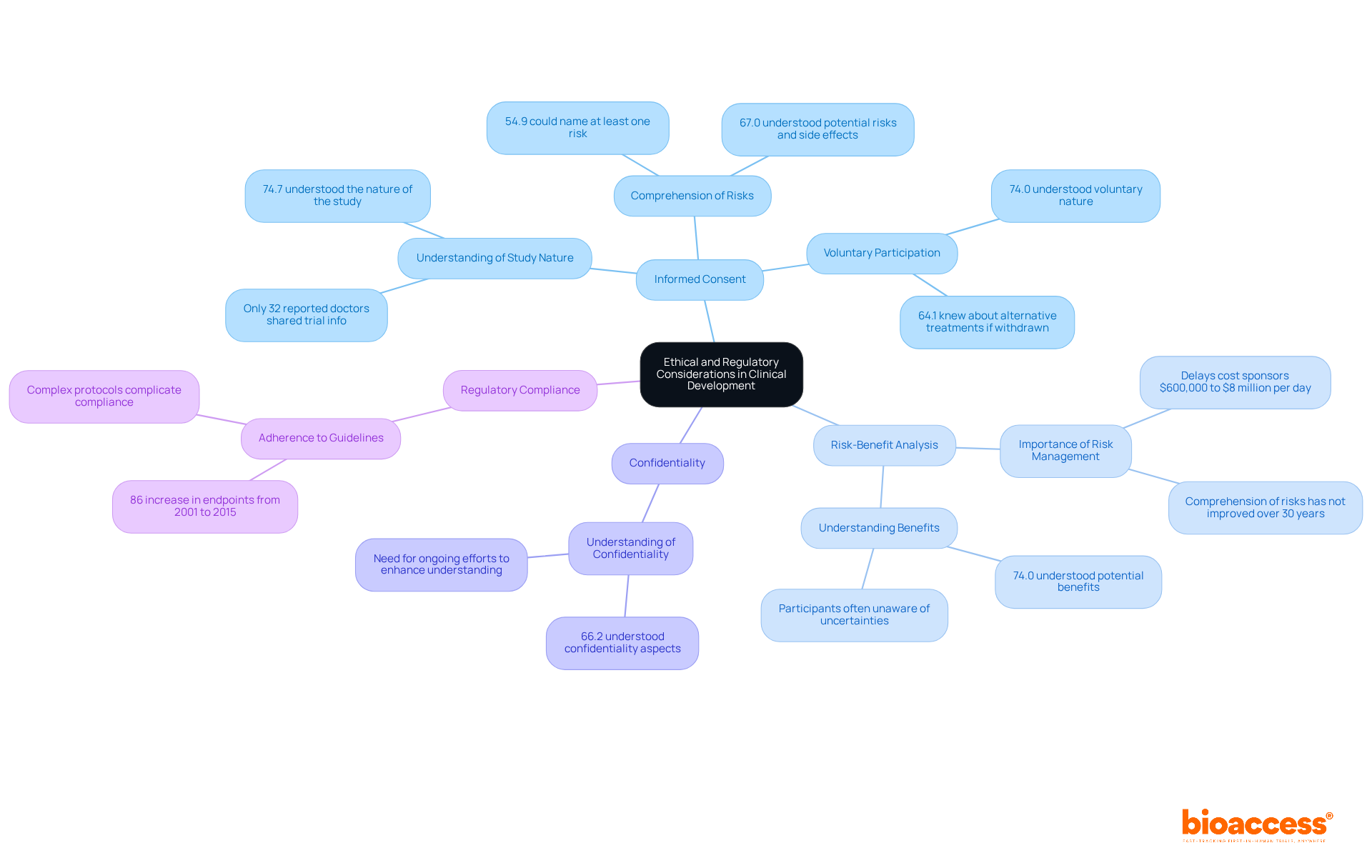
Ethical factors in medical development are crucial for ensuring what is clinical development by safeguarding the safety and rights of individuals involved in clinical research. Key ethical principles include:
Informed Consent: Participants must be fully informed about the study's purpose, procedures, risks, and benefits before agreeing to participate. Research indicates that while 74.7% of individuals understood the nature of the study, many struggled to name specific risks. This highlights a significant gap in comprehension that needs to be addressed. Furthermore, only 32% of patients reported that their doctors had shared information about clinical trials with them, underscoring the need for improved communication from healthcare providers.
Risk-Benefit Analysis: Researchers must ensure that the potential benefits of the research outweigh the risks to those involved. A systematic review revealed that comprehension of risks and benefits has not advanced over the past 30 years, indicating a persistent challenge in effectively conveying these critical aspects to individuals. Delays in clinical trials can cost sponsors between $600,000 and $8 million for each day of delay, emphasizing the importance of efficient risk management.
Confidentiality: Safeguarding the privacy of individuals and the confidentiality of their data is essential. Approximately 66.2% of individuals grasped confidentiality aspects, yet ongoing efforts are necessary to enhance this understanding further.
Regulatory Compliance: Adhering to guidelines set forth by regulatory bodies, such as the FDA, is essential for maintaining the integrity of the research process. The overall count of endpoints in research protocols has risen by 86% from 2001 to 2015, complicating adherence and subject comprehension.
These ethical and regulatory frameworks not only safeguard participants but also boost the credibility of medical research, fostering public trust in the research community. As the landscape of clinical trials evolves, continuous improvement in informed consent practices and risk communication will be vital for ethical research conduct.
Clinical development stands as a cornerstone in healthcare, ensuring that new medical interventions undergo rigorous testing for safety and efficacy before reaching patients. This systematic process not only bridges the gap between laboratory research and clinical application but also plays a pivotal role in enhancing patient outcomes and informing best practices in medical care.
The article outlines key phases of clinical development, from exploratory studies in Phase 0 to post-marketing surveillance in Phase IV. Each phase serves a distinct purpose, addressing specific inquiries about new treatments while underscoring the importance of collaboration among stakeholders. Furthermore, ethical considerations underpinning clinical trials, such as informed consent and risk-benefit analysis, are highlighted, reinforcing the necessity for transparency and participant safety in research.
The significance of clinical development cannot be overstated. It is essential for driving innovation in medicine and ensuring that only safe and effective treatments are made available to patients. As the landscape of clinical trials evolves, embracing advancements like real-world evidence and decentralized studies will be vital for improving recruitment and retention. This evolution ultimately leads to more effective healthcare solutions. Stakeholders in this field must prioritize ethical practices and regulatory compliance, fostering trust and credibility in the research community while paving the way for future breakthroughs in medical science.
What is clinical development?
Clinical development is a systematic process that involves researching and testing new medical interventions, such as drugs, devices, and therapies, to assess their safety and efficacy for human use.
Why is clinical development important?
Clinical development is crucial in the healthcare sector as it acts as a vital link between laboratory research and practical application, aiming to produce reliable data that supports regulatory approval and informs practice, ultimately enhancing patient outcomes.
What are the phases of the medical development process?
The medical development process is organized into multiple phases, each designed to address specific inquiries about the intervention's impact on health and illness.
What challenges do experimental drugs face in clinical development?
Statistics reveal that only 10% of experimental drugs receive FDA approval, and around 80% of research studies encounter delays or shutdowns due to recruitment challenges.
How does real-world evidence (RWE) impact clinical development?
The incorporation of RWE has shown an increase in patient recruitment and retention by up to 20%, providing insights into patient behavior that enhance study designs.
What are decentralized studies, and how have they been influenced by the COVID-19 pandemic?
Decentralized studies allow for quicker enrollment and increased diversity among participants, aligning studies more closely with real-world patient experiences, which has been accelerated by the COVID-19 pandemic.
What advantages does bioaccess® provide in clinical development?
Bioaccess® enables treatment-naive cardiology or neurology cohorts to enroll 50% faster than Western sites and achieves significant cost savings of $25K per patient with FDA-ready data.
How do partnerships in clinical development enhance effectiveness?
Partnerships, such as the one with Welwaze Medical Inc. for the Celbrea® medical device introduction in Colombia, help overcome regulatory and market access obstacles, enhancing the effectiveness of studies.
What financial impact does integrating RWE into clinical studies have on the pharmaceutical sector?
The pharmaceutical sector could save up to $50 billion each year by integrating RWE into clinical studies, emphasizing the financial impact of these strategies on clinical advancement.