


FDA Form 483 serves as a critical document issued by the FDA, signaling potential compliance issues identified during inspections of clinical research organizations. Such issues, if left unaddressed, can result in serious regulatory consequences, underscoring the form's significance in the clinical research landscape. Understanding and responding effectively to the observations noted in a Form 483 is essential for maintaining the integrity of clinical trials and ensuring participant safety. This document reflects a company’s adherence to Good Clinical Practice, which can significantly impact product approval timelines.
In the ever-evolving Medtech landscape, the role of organizations in addressing compliance challenges cannot be overstated. Companies must recognize the importance of these observations and take proactive steps to rectify any identified issues. By doing so, they not only safeguard their operations but also enhance the overall quality of clinical research. Collaboration among stakeholders is key to navigating these challenges effectively.
In conclusion, the importance of addressing FDA Form 483 observations cannot be overlooked. Companies must prioritize compliance to ensure the integrity of clinical trials and the safety of participants. As the landscape of clinical research continues to change, staying vigilant and responsive to regulatory feedback will be crucial for maintaining a competitive edge.
Understanding the intricacies of FDA Form 483 is crucial for any organization involved in clinical research. This document, issued by the U.S. Food and Drug Administration, serves as a pivotal warning sign, indicating potential compliance issues that could jeopardize the integrity of clinical trials and the safety of participants.
As organizations navigate the complex landscape of regulatory compliance, the question arises: how can they effectively respond to and mitigate the risks associated with receiving an FDA 483?
This article delves into the significance of this form, its implications for clinical research, and the strategies organizations can employ to ensure adherence to regulatory standards.
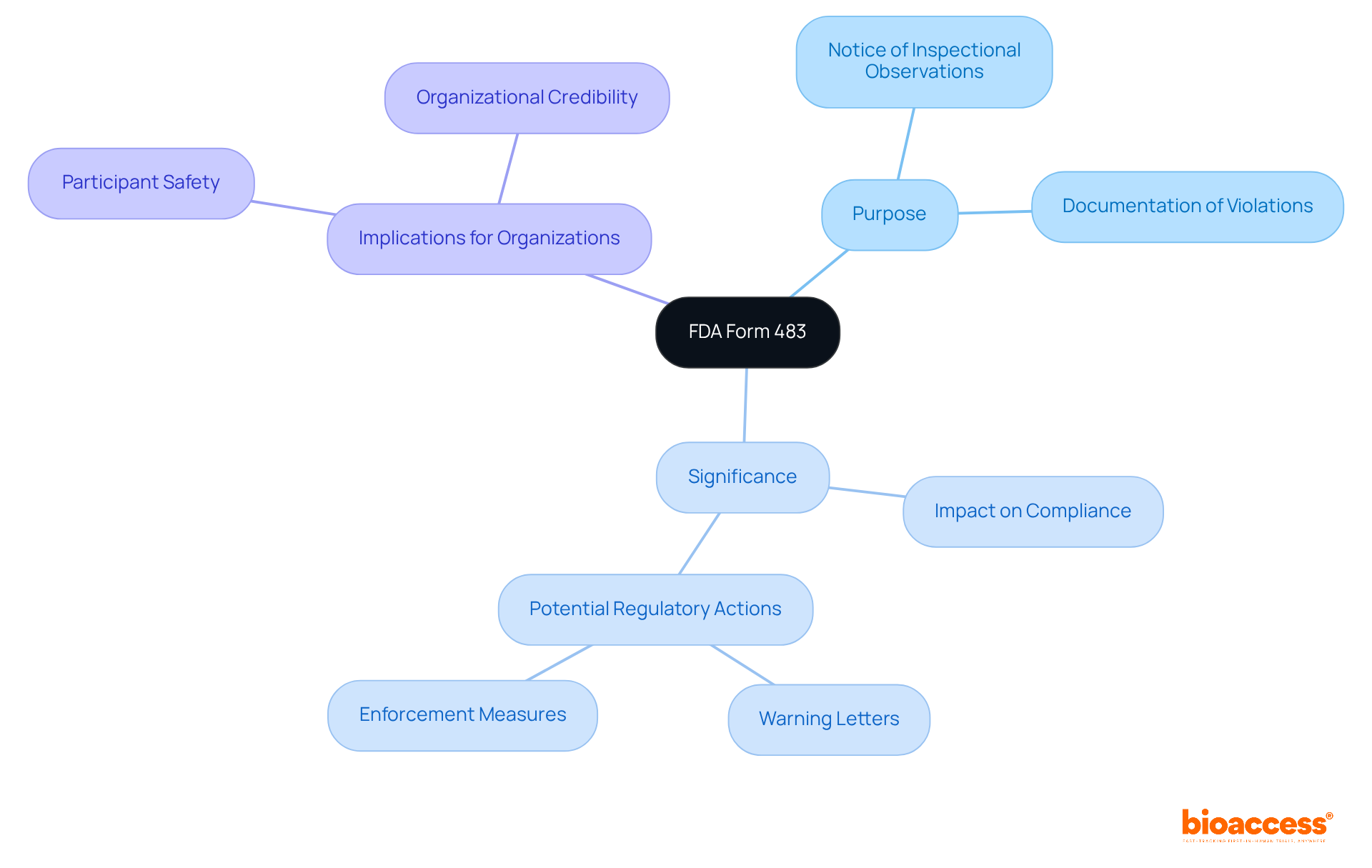
FDA Form 483, officially referred to as the 'Notice of Inspectional Observations,' is a critical document issued by the U.S. Food and Drug Administration (FDA) to a firm's management upon the conclusion of an inspection. This form meticulously documents any conditions observed by FDA investigators that may suggest violations of the Food, Drug, and Cosmetic Act, along with related regulations. Its significance lies in its role as an initial alert, highlighting potential adherence issues that could lead to more serious regulatory actions, such as warning letters or enforcement measures.
Understanding what is 483 fda is essential for clinical research organizations and sponsors, as it directly affects the integrity of clinical trials and the safety of participants. By recognizing the implications of this form, organizations can proactively address compliance challenges, thereby fostering a culture of quality and safety in their operations. This awareness not only safeguards participant welfare but also enhances the organization's credibility and trustworthiness in the Medtech landscape.
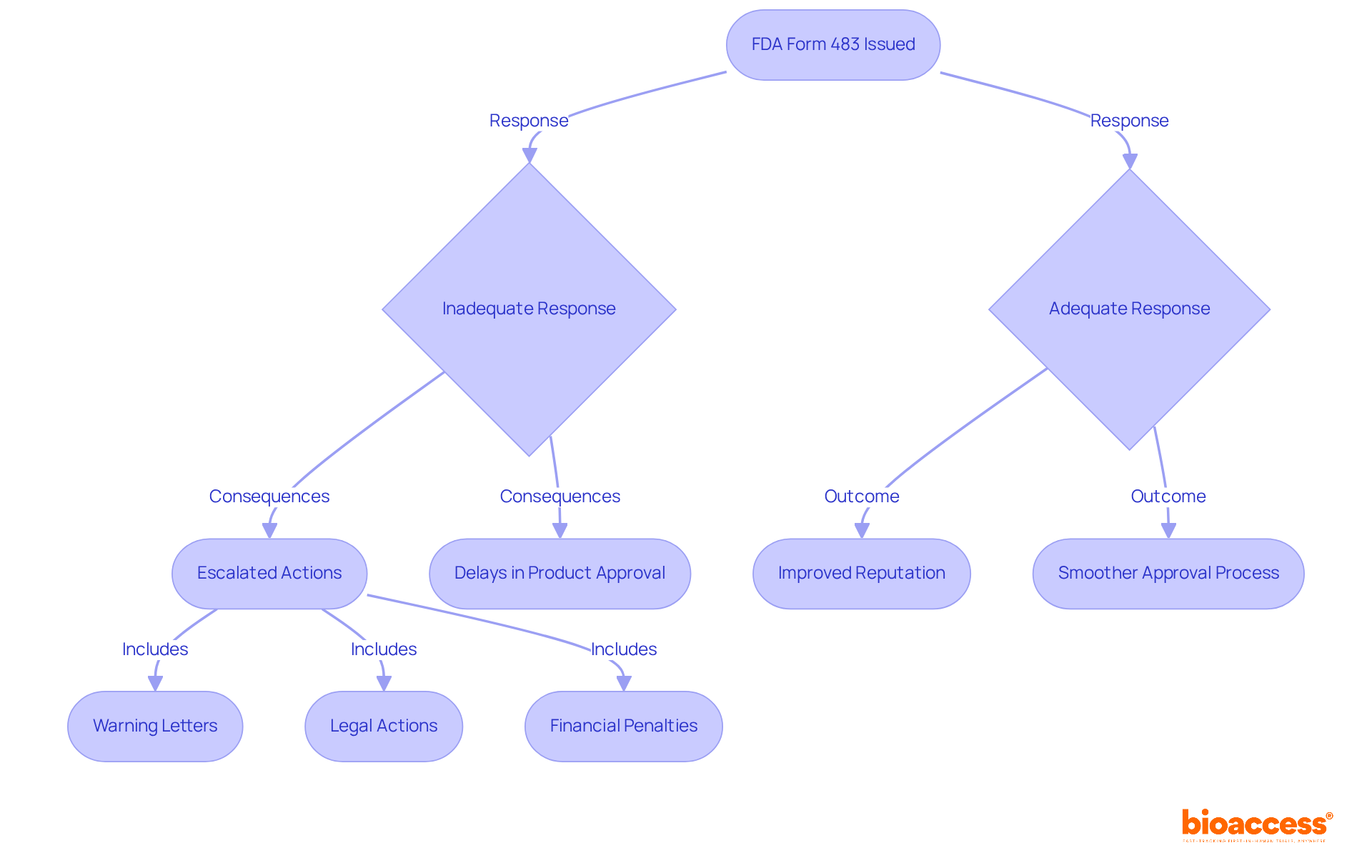
To understand what is 483 FDA, one must recognize that the issuance of an FDA Form 483 serves as a pivotal indicator of a company's compliance status, reflecting the FDA's evaluation of adherence to Good Clinical Practice (GCP) and other regulatory standards. Understanding what is 483 FDA can highlight the profound implications of receiving such a notice, including heightened scrutiny, potential delays in product approvals, and reputational damage. For instance, companies that fail to adequately address remarks noted in what is 483 FDA may face escalated actions, such as warning letters, which can result in severe consequences, including legal actions and financial penalties.
Statistics indicate that insufficient replies to what is 483 FDA remarks can significantly impact the approval schedules of New Drug Applications (NDA); unresolved issues may necessitate re-inspections, further postponing the process. Organizations must prioritize these insights, implementing corrective measures promptly to mitigate risks. A proactive approach not only demonstrates a commitment to compliance but also aids in preserving relationships with stakeholders and maintaining operational integrity.
Industry instances illustrate that firms effectively responding to what is 483 FDA remarks can avoid escalation and improve their reputation with regulatory agencies, ultimately facilitating smoother pathways to product approval.
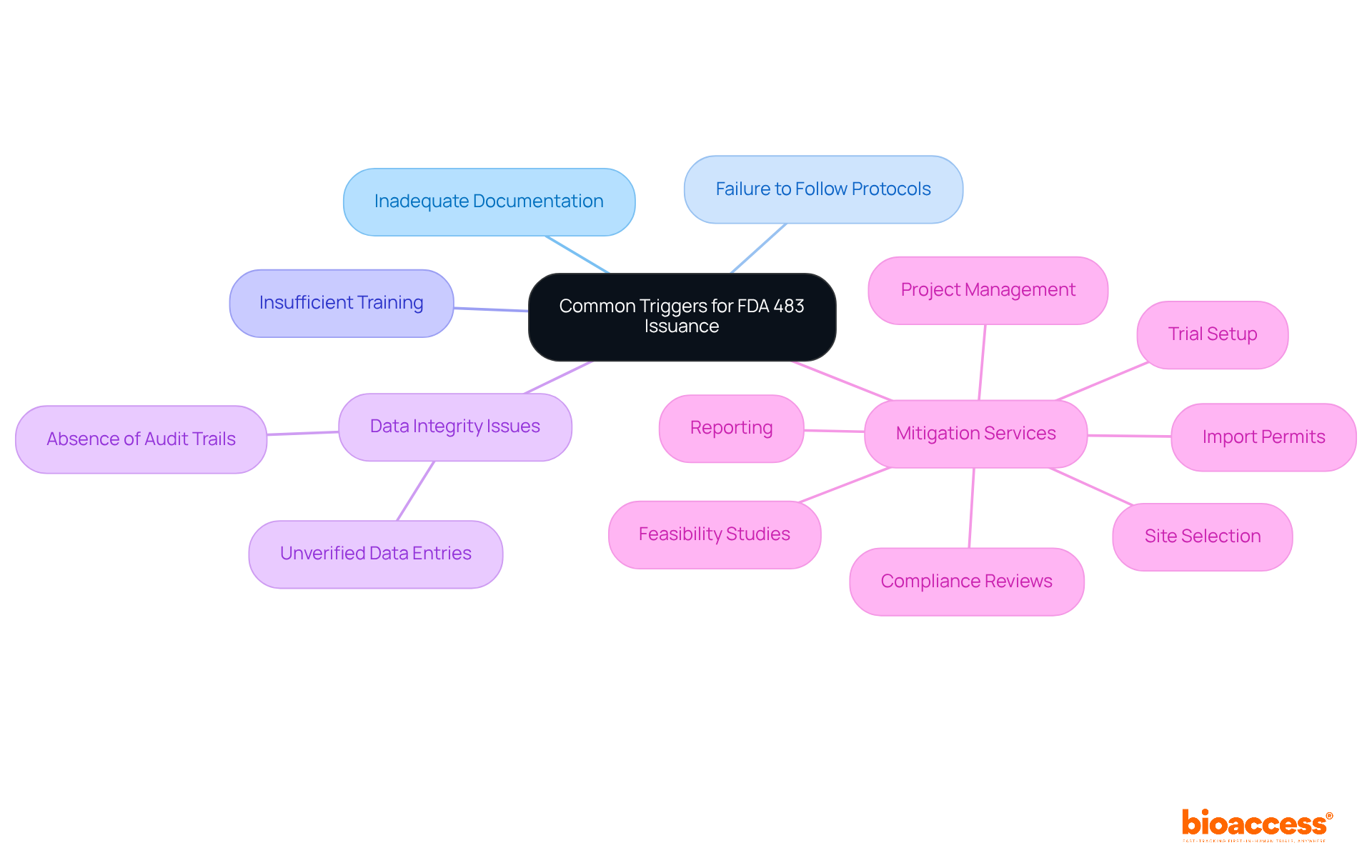
Inadequate documentation practices, failure to follow established protocols, and insufficient training of personnel involved in clinical trials are common triggers for what is 483 fda. Other prevalent findings involve issues related to data integrity, such as unverified data entries or the absence of proper audit trails. For instance, if an investigator fails to document adverse events accurately, it may lead to a Form 483. By understanding these triggers, organizations can implement robust training programs and quality assurance measures to minimize the risk of receiving a Form 483, which is what is 483 fda, during inspections.
Additionally, comprehensive clinical trial management services, such as those provided by bioaccess, play a crucial role in mitigating these risks. Services include:
This thorough approach enhances compliance and significantly reduces the likelihood of receiving a Form 483, which raises the question of what is 483 fda.
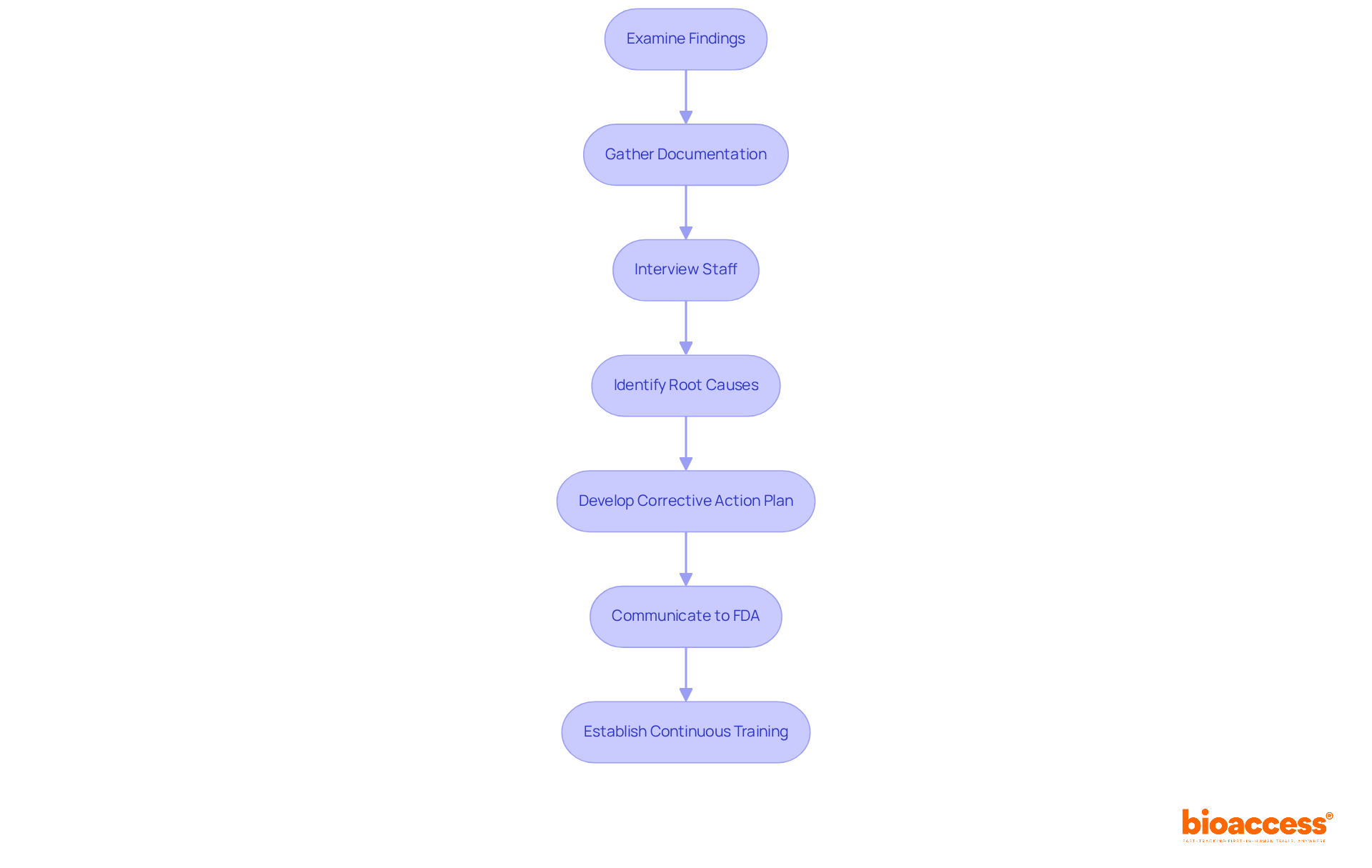
Addressing FDA Form 483 comments necessitates a systematic method. Organizations should first carry out a comprehensive examination of the findings recorded in what is 483 fda. This involves:
Once the investigation is complete, firms should develop a corrective action plan that addresses each observation, outlining specific steps to rectify the issues and prevent recurrence. It is essential to communicate this plan to the FDA by addressing what is 483 fda within the stipulated 15 business days. Furthermore, organizations should establish continuous training and monitoring to guarantee adherence to FDA regulations going forward. By taking these proactive measures, firms can demonstrate their commitment to regulatory compliance and mitigate the risk of further actions from the FDA.
Understanding FDA Form 483 is crucial for any organization involved in clinical research. This document serves as a formal notification from the FDA, highlighting potential compliance issues that could jeopardize the integrity of clinical trials and the safety of participants. Recognizing its significance allows organizations to take proactive measures to ensure adherence to regulations, ultimately fostering a culture of quality and safety that enhances credibility in the Medtech field.
The implications of receiving an FDA Form 483 are profound, including:
It is essential to address the observations noted in the form; neglecting these can lead to escalated regulatory actions and significant consequences. Common triggers for FDA 483 issuance, such as inadequate documentation and insufficient staff training, underscore the necessity for robust compliance strategies.
In light of these insights, organizations are encouraged to adopt effective response strategies to FDA Form 483 findings. This includes:
By embracing these best practices, firms can not only mitigate risks but also demonstrate their commitment to regulatory compliance, thereby paving the way for smoother pathways to product approval and maintaining trust with stakeholders in the clinical research community.
What is FDA Form 483?
FDA Form 483, officially known as the 'Notice of Inspectional Observations,' is a document issued by the U.S. Food and Drug Administration (FDA) to a firm's management at the end of an inspection. It details any conditions observed that may indicate violations of the Food, Drug, and Cosmetic Act and related regulations.
What is the purpose of FDA Form 483?
The purpose of FDA Form 483 is to serve as an initial alert to organizations about potential adherence issues that could lead to more serious regulatory actions, such as warning letters or enforcement measures.
Why is understanding FDA Form 483 important for clinical research organizations?
Understanding FDA Form 483 is crucial for clinical research organizations and sponsors because it directly impacts the integrity of clinical trials and the safety of participants. Recognizing its implications helps organizations proactively address compliance challenges.
How does FDA Form 483 affect participant safety and organizational credibility?
By being aware of FDA Form 483 and its implications, organizations can foster a culture of quality and safety, which safeguards participant welfare and enhances their credibility and trustworthiness in the Medtech landscape.