


FDA 483 is a pivotal document issued by the U.S. Food and Drug Administration (FDA) following an inspection. It indicates potential violations of FDA regulations that could significantly impact the safety, efficacy, and quality of clinical research. This document serves as a critical communication tool and an early warning system, enabling organizations to proactively address compliance issues. By doing so, they can maintain the integrity of their clinical trials and ensure adherence to regulatory standards.
Understanding the nuances of FDA 483 is essential for anyone involved in clinical research. This pivotal document serves as a crucial indicator of compliance with regulatory standards. By shedding light on specific observations made during FDA inspections, it highlights potential areas of concern and offers organizations a chance to rectify issues before they escalate into serious repercussions.
However, the challenge remains: how can research entities effectively navigate the complexities of FDA 483 findings to ensure both compliance and the integrity of their studies?
What is FDA 483? It is a critical document that the U.S. Food and Drug Administration (FDA) issues at the conclusion of an inspection. It signals to a firm’s management that certain conditions observed may violate FDA regulations. This form serves as a vital communication tool, outlining specific insights that can greatly impact the safety, efficacy, and quality of medical research. Its importance lies in functioning as an early warning system, enabling organizations to rectify potential compliance issues before they escalate into severe regulatory actions, such as warning letters or enforcement actions.
In 2023, the FDA referenced 21 CFR 211.84 for not testing and approving components 37 times, highlighting the essential nature of compliance in trials. Observations noted on Form 483 should be addressed promptly, as timely responses can mitigate regulatory risks and foster a cooperative relationship between researchers and regulators. Organizations that effectively respond to Form 483 observations can enhance their compliance posture and improve study integrity.
Understanding what is FDA 483 is significant, as it transcends mere adherence and is crucial for research organizations (CROs) and sponsors to thoroughly comprehend its consequences. The form acts as a reminder of the necessity for strict compliance with regulatory standards, especially considering recent examinations following negative occurrences in research trials. By prioritizing compliance and cultivating a culture of conscience, CROs can better safeguard research subjects and ensure the successful execution of medical studies.
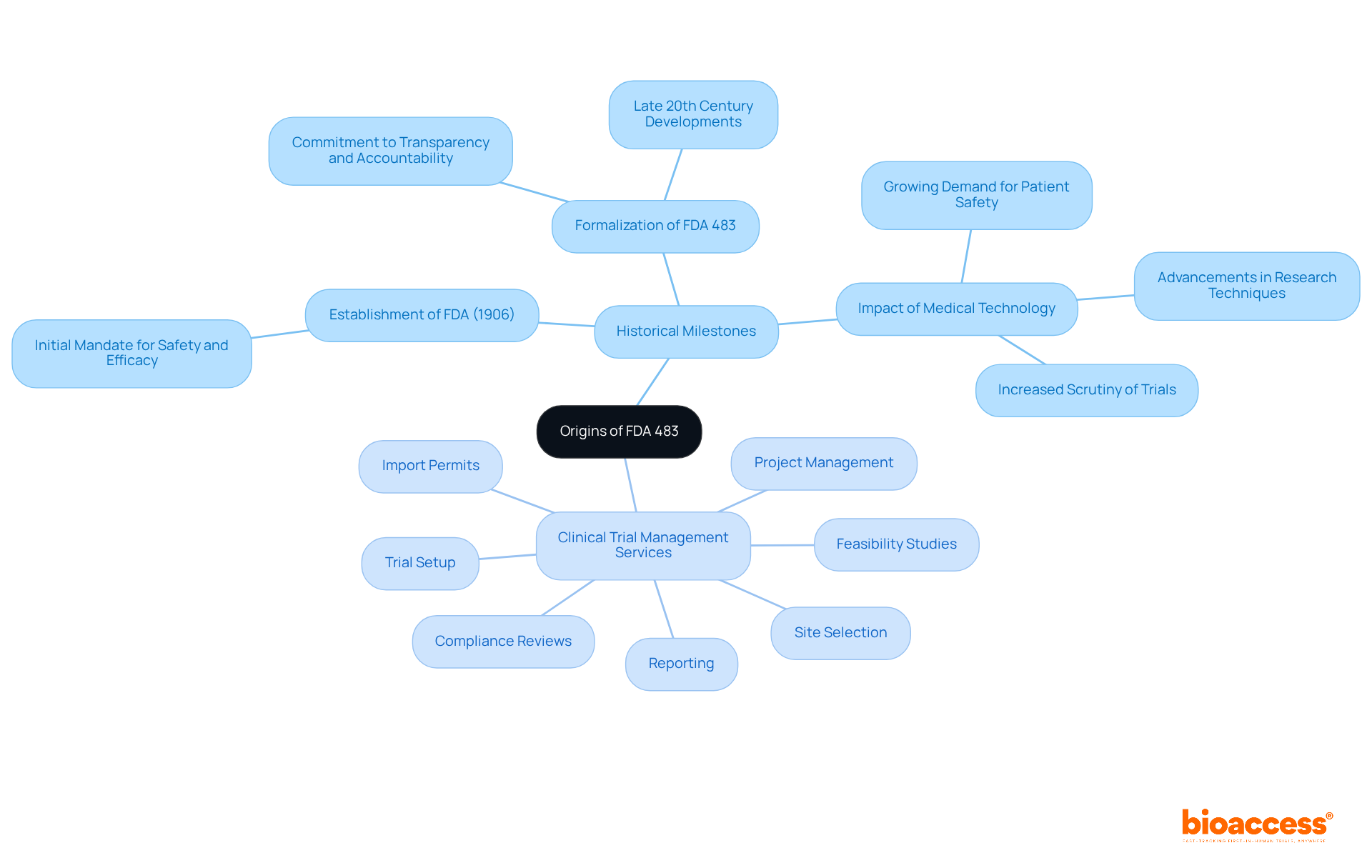
The origins of FDA Form 483 can be traced back to the establishment of the FDA in 1906, when the agency was tasked with ensuring the safety and efficacy of food and drugs. As the complexity of medical research evolved over the decades, so too did the necessity for a structured approach to inspections. The formalization of FDA 483 in its current form occurred in the late 20th century, reflecting the FDA's unwavering commitment to transparency and accountability in the regulatory process. This development has been shaped by several factors, including advancements in medical technology, increased scrutiny of trials, and the growing demand for enhanced patient safety. Today, what is FDA 483 serves as a critical component of the FDA's inspection process, providing a vital mechanism for investigators to communicate their findings effectively.
In this context, comprehensive clinical trial management services offered by bioaccess are essential for ensuring adherence to FDA regulations and navigating the complexities of clinical research. These services include:
By leveraging these services, organizations can address key challenges in the Medtech landscape, ultimately fostering a collaborative environment that prioritizes patient safety and regulatory compliance. As the clinical research field continues to evolve, the importance of strategic partnerships and expert guidance cannot be overstated. Organizations must consider how they can enhance their trial management processes to align with FDA standards and improve overall outcomes.
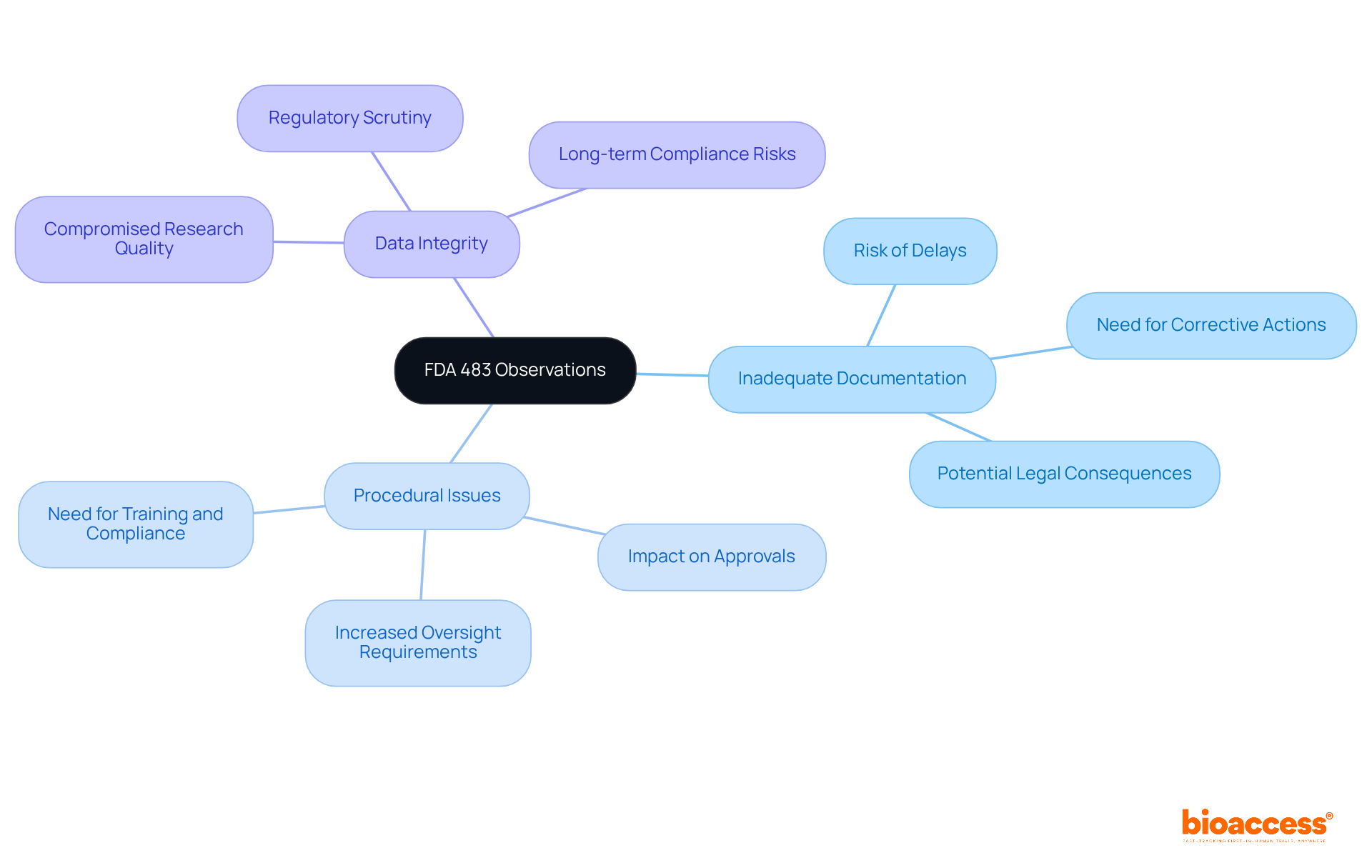
Key features of what is FDA 483 include specific findings noted by FDA inspectors during evaluations. These insights typically fall into categories such as:
Each assessment reflects the investigator's judgment concerning factors that may compromise the quality of clinical research. For instance, Gland Pharma has observed that procedural issues can significantly delay or affect approvals for sterile active pharmaceutical ingredients (APIs), underscoring the necessity of addressing these remarks promptly.
The implications of these insights can be profound; they may lead to further investigations, the issuance of warning letters, or even legal actions if not promptly addressed. Understanding these characteristics is crucial for organizations aiming to implement corrective actions and maintain compliance with FDA regulations, particularly in relation to what is FDA 483.
By utilizing comprehensive trial management services—such as feasibility studies, site selection, compliance reviews, and efficient trial setup—companies can proactively mitigate potential issues that may arise during FDA inspections. As noted by Andrea Corona, the FDA is currently grappling with investigator vacancies, complicating oversight and increasing the risk of repeated findings.
By fostering a culture of quality and accountability, alongside robust project management and reporting practices, companies can minimize risks associated with these insights and enhance the integrity of their clinical research processes. This, in turn, contributes to job creation and economic growth within local economies.
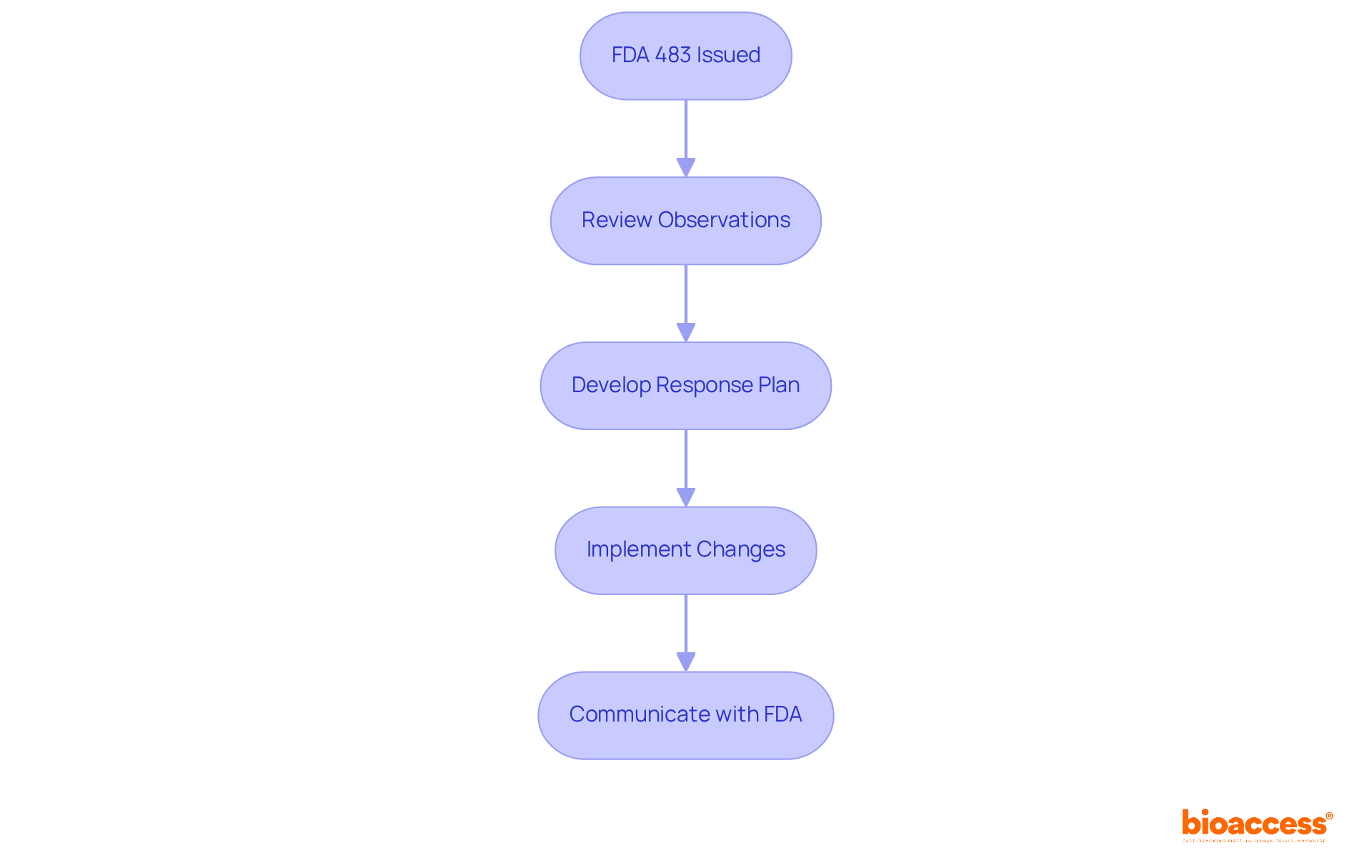
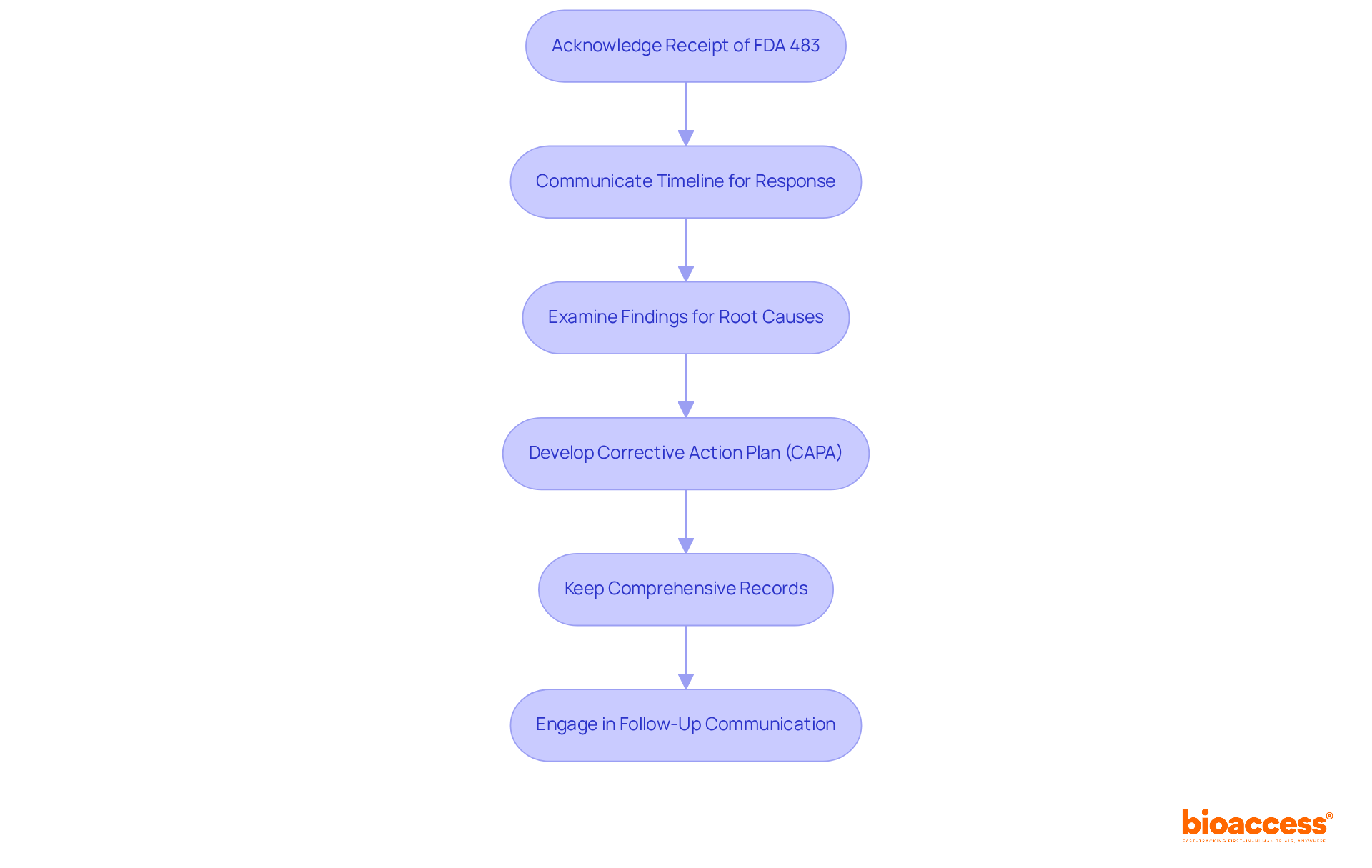
Understanding what is FDA 483 necessitates a systematic approach to effectively respond to all remarks. To begin, promptly acknowledge receipt of the FDA 483 and communicate with the FDA regarding the timeline for your response, ideally within 15 business days. Next, conduct a thorough examination of each finding to identify root causes. Following this, develop a corrective action plan (CAPA) that outlines specific measures to address the findings, including clear implementation timelines. It is also essential to keep comprehensive records of all actions taken in reaction to the findings, ensuring that documentation is clear and readily accessible. Finally, engage in follow-up communication with the FDA to verify that corrective actions have been successfully implemented.
Understanding what is FDA 483 and the serious consequences, such as warning letters, product recalls, and reputational damage, that can arise from receiving it is crucial. Therefore, maintaining transparent communication with the FDA during the NDA review process is vital for addressing potential effects stemming from what is FDA 483 findings. By adhering to these best practices, organizations can demonstrate their commitment to regulatory compliance and enhance their overall quality management systems. Successful compliance strategies, particularly in 2025, underscore the importance of timely and organized responses, alongside the implementation of CAPA, which has proven effective in improving compliance rates among organizations trying to understand what is FDA 483 observations.
Understanding the role of FDA 483 is essential for any organization involved in clinical research. This document highlights potential compliance issues and serves as a proactive measure to ensure that research quality, safety, and efficacy are maintained. By recognizing the significance of FDA 483, organizations can better navigate the complexities of regulatory compliance and foster a culture that prioritizes accountability and quality in clinical trials.
Throughout this article, key points have been discussed, including:
The evolution of this form reflects the FDA's commitment to transparency and safety in medical research. Moreover, the implications of FDA 483 findings can lead to severe consequences if not addressed promptly, emphasizing the importance of timely and thorough responses.
Ultimately, the significance of FDA 483 extends beyond mere compliance; it is a cornerstone of integrity in clinical research that safeguards participants and enhances the credibility of the research community. Organizations must take proactive steps to understand and implement effective compliance strategies to ensure they are not only meeting regulatory requirements but also contributing to the advancement of safe and effective medical treatments. Prioritizing these practices can lead to improved outcomes and foster a healthier environment for innovation in drug development.
What is FDA 483?
FDA 483 is a critical document issued by the U.S. Food and Drug Administration (FDA) at the conclusion of an inspection. It indicates to a firm’s management that certain observed conditions may violate FDA regulations.
Why is FDA 483 important in clinical research?
FDA 483 serves as a vital communication tool that outlines specific insights impacting the safety, efficacy, and quality of medical research. It functions as an early warning system, allowing organizations to address potential compliance issues before they lead to severe regulatory actions.
What does the FDA emphasize regarding compliance in clinical trials?
In 2023, the FDA referenced 21 CFR 211.84 for not testing and approving components 37 times, highlighting the essential nature of compliance in trials.
How should organizations respond to observations noted on Form 483?
Observations noted on Form 483 should be addressed promptly, as timely responses can help mitigate regulatory risks and foster a cooperative relationship between researchers and regulators.
What benefits do organizations gain by effectively responding to Form 483 observations?
Organizations that effectively respond to Form 483 observations can enhance their compliance posture and improve the integrity of their studies.
Why is understanding FDA 483 significant for research organizations and sponsors?
Understanding FDA 483 is crucial for research organizations (CROs) and sponsors as it highlights the importance of strict compliance with regulatory standards, especially in light of recent examinations following negative occurrences in research trials.
How can CROs safeguard research subjects and ensure successful medical studies?
By prioritizing compliance and cultivating a culture of conscience, CROs can better safeguard research subjects and ensure the successful execution of medical studies.