


The IND study, or Investigational New Drug trial, represents a pivotal process that empowers researchers to seek FDA approval for administering new substances to human participants, primarily to evaluate their safety and efficacy. This article underscores the essential role of IND studies in propelling medical innovations, as they establish a regulatory framework that guarantees investigational therapies adhere to safety standards. Consequently, this framework facilitates the development of effective treatments for patients, highlighting the significance of these trials in the broader context of clinical research.
Understanding the intricacies of Investigational New Drug (IND) studies is essential for grasping their pivotal role in the clinical trial landscape. These studies serve as a formal request to the FDA for human testing of new therapies and act as a crucial bridge between laboratory research and real-world medical application. As the demand for innovative treatments grows, the efficiency and effectiveness of the IND process come under scrutiny. This raises a critical question: how do IND studies ensure both patient safety and the rapid advancement of medical innovation?
An Investigational New Product (IND) trial represents a pivotal component of the clinical trial process, serving as a formal petition to the Food and Drug Administration (FDA) for authorization to administer an investigational substance or biological product to human participants. The primary objective of an IND investigation is to ascertain the safety of the investigational drug for initial human testing, while simultaneously providing critical data that underpins its scientific validity. These investigations are indispensable for advancing medical innovations, effectively bridging laboratory research and practical application. They empower researchers to rigorously assess the safety and efficacy of novel therapies within human populations.
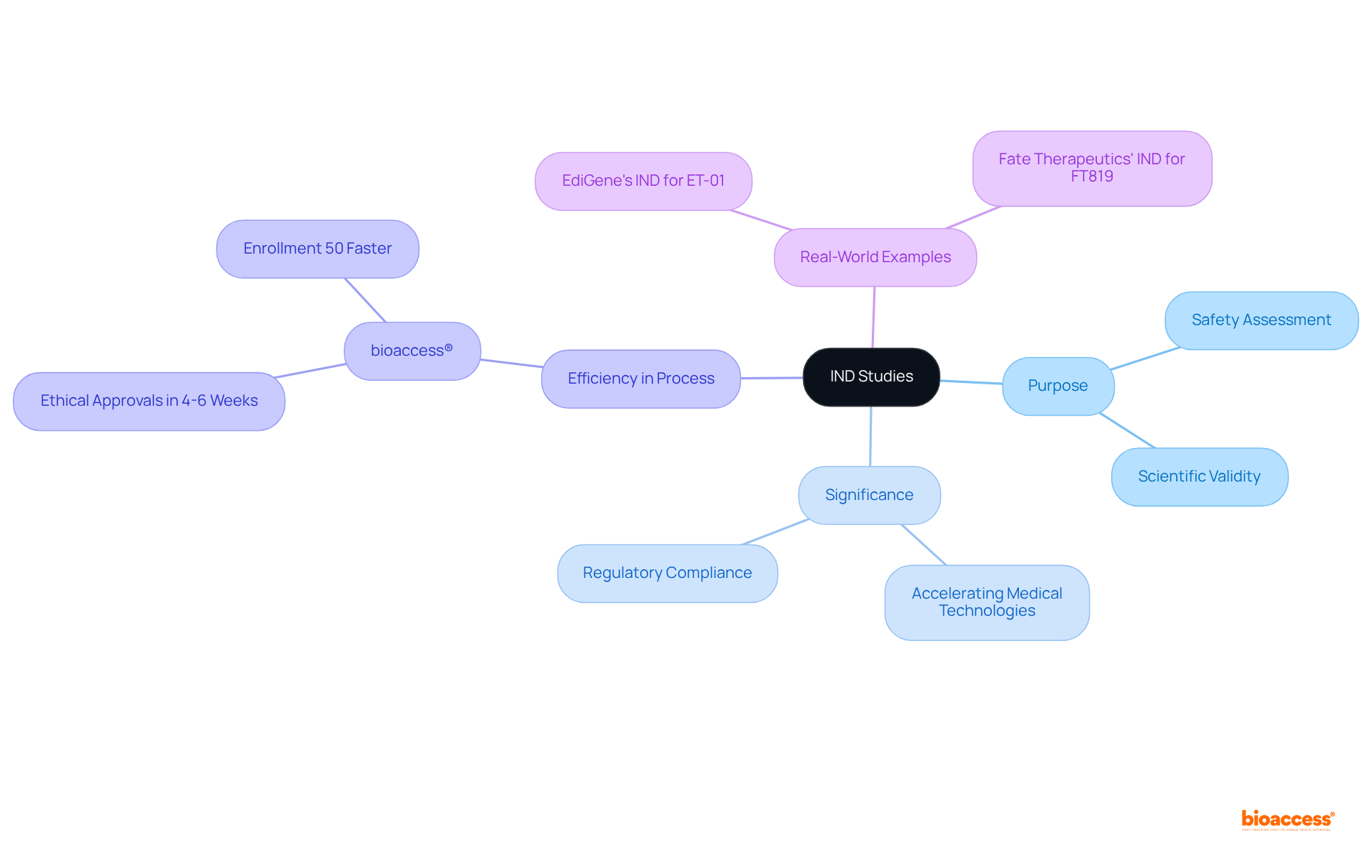
As we approach 2025, understanding what is IND study is crucial due to its role in accelerating the advancement of medical technologies. For example, companies like bioaccess® leverage their extensive expertise, honed over 15 years in clinical research, to streamline the IND process, achieving ethical approvals in a mere 4-6 weeks and ensuring participant enrollment is 50% faster than traditional markets. This remarkable efficiency not only hastens the timeline for delivering innovative therapies to patients but also enhances the overall safety evaluations of new medications.
Real-world instances illustrate the profound impact of IND research on drug safety and efficacy assessments. For instance, EdiGene's IND application for ET-01, a modified cell therapy for beta-thalassemia developed using CRISPR-Cas9 technology, received approval from China's National Medical Products Administration, allowing the company to advance its trial development. Similarly, Fate Therapeutics secured IND approval from the FDA for FT819, a CAR T-cell therapy targeting acute lymphoblastic leukemia (ALL), underscoring the essential role of these investigations in advancing therapeutic alternatives.
FDA officials have consistently emphasized what is IND study, asserting that it is critical for ensuring that investigational therapies comply with safety standards prior to entering clinical trials. This regulatory framework not only safeguards participants but also cultivates innovation within the medical field, ultimately culminating in the development of effective treatments that can significantly enhance patient outcomes.
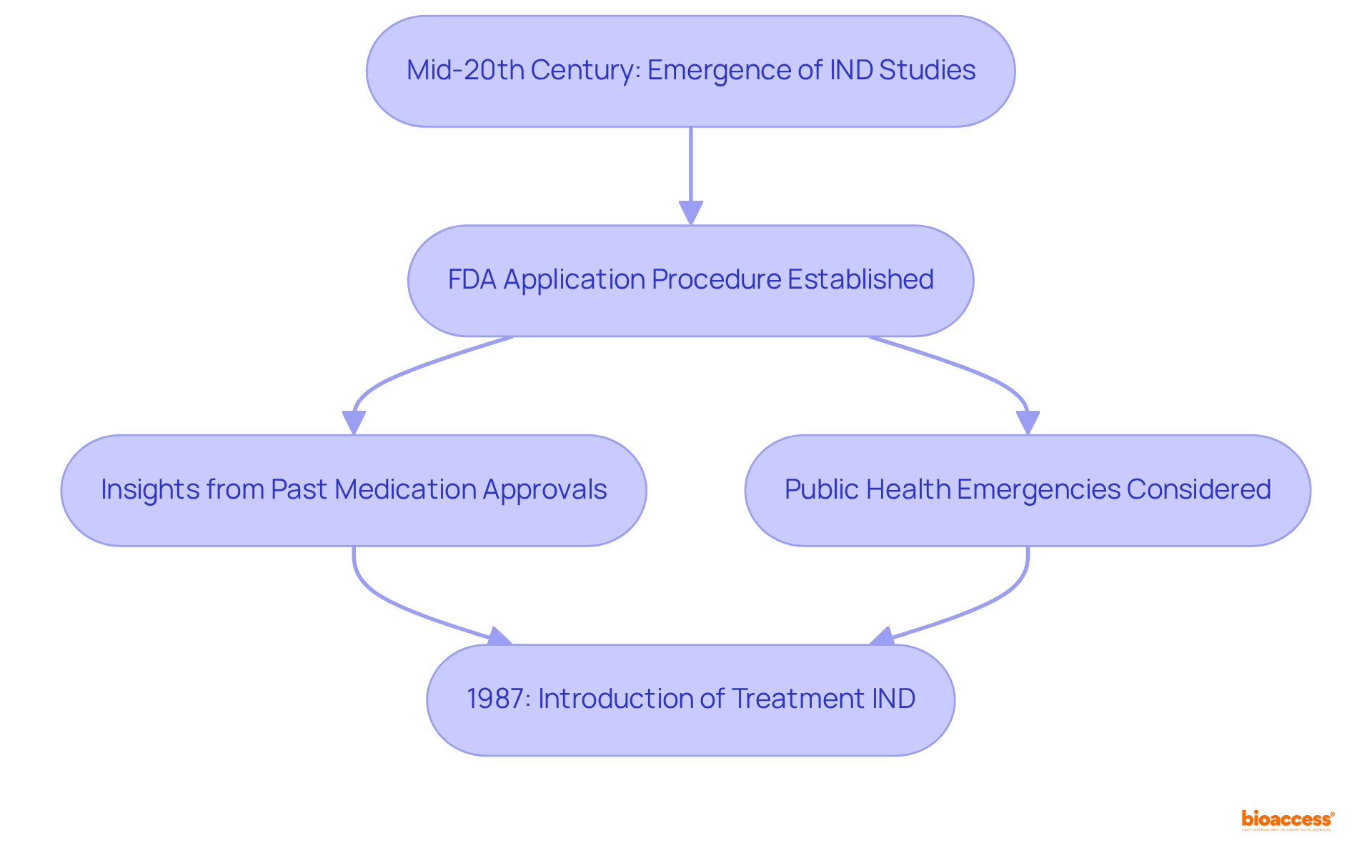
What is IND study refers to the concept that emerged in the mid-20th century in response to the pressing need for comprehensive safety evaluations of new substances prior to human testing. To understand what is IND study, the FDA instituted the application procedure to ensure investigational substances met established safety and efficacy standards. The evolution of the IND process over time can be understood through what is IND study, incorporating insights gained from past medication approvals and public health emergencies. Notably, the introduction of the Treatment IND in 1987 expanded access to promising therapies for patients with serious conditions, reflecting a significant shift towards patient-centered care in the development of medications.
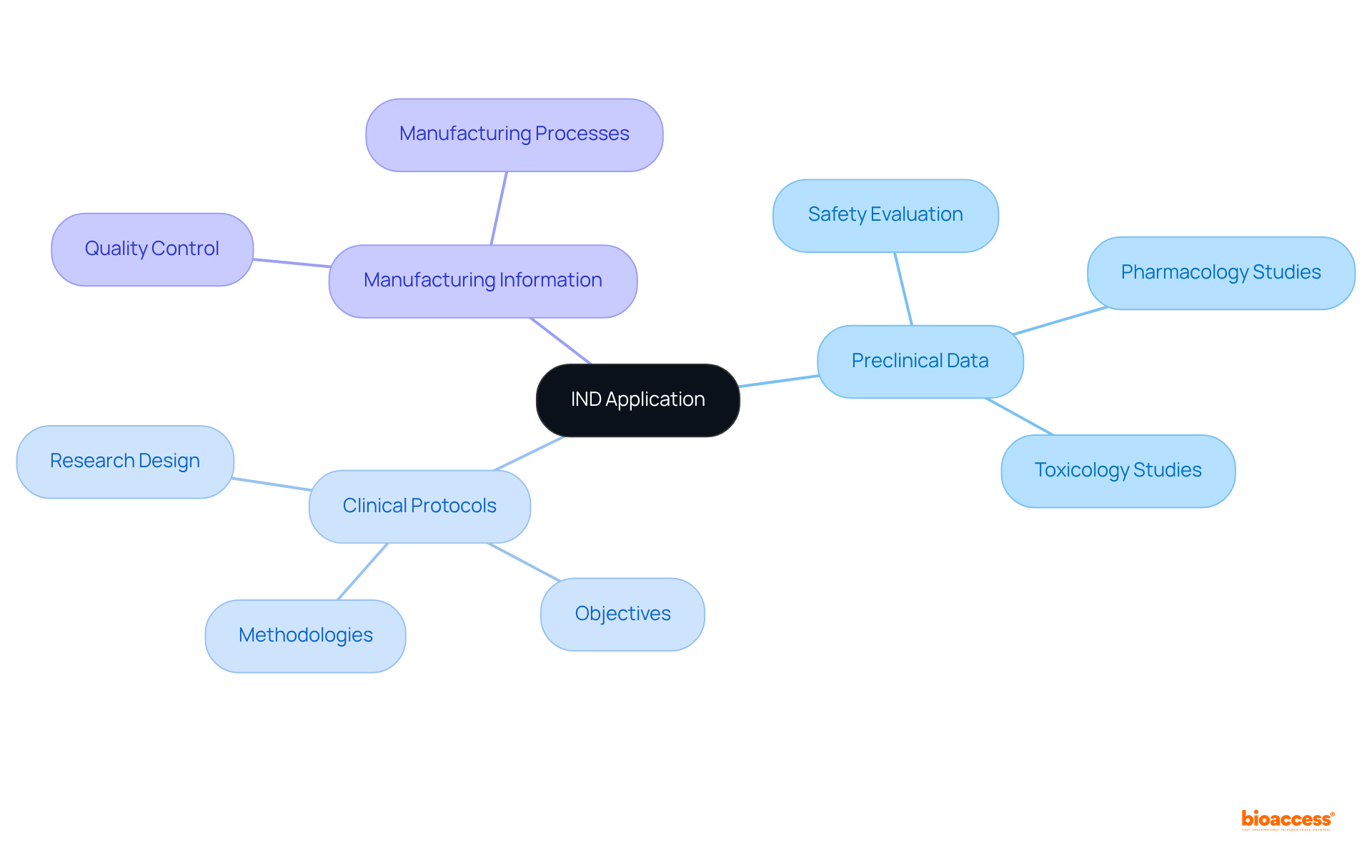
An IND application is essential for clinical research, requiring comprehensive information across three key areas:
Preclinical data encompasses animal research that evaluates the medication's pharmacology and toxicology, establishing a solid foundation for its safety in humans. Clinical protocols outline the research design, objectives, and methodologies for assessing the medication's effects. Additionally, the application must detail the medication's manufacturing processes to ensure quality control.
The FDA conducts an evaluation of the IND application within 30 days, scrutinizing the safety of the proposed research and the qualifications of the research team.
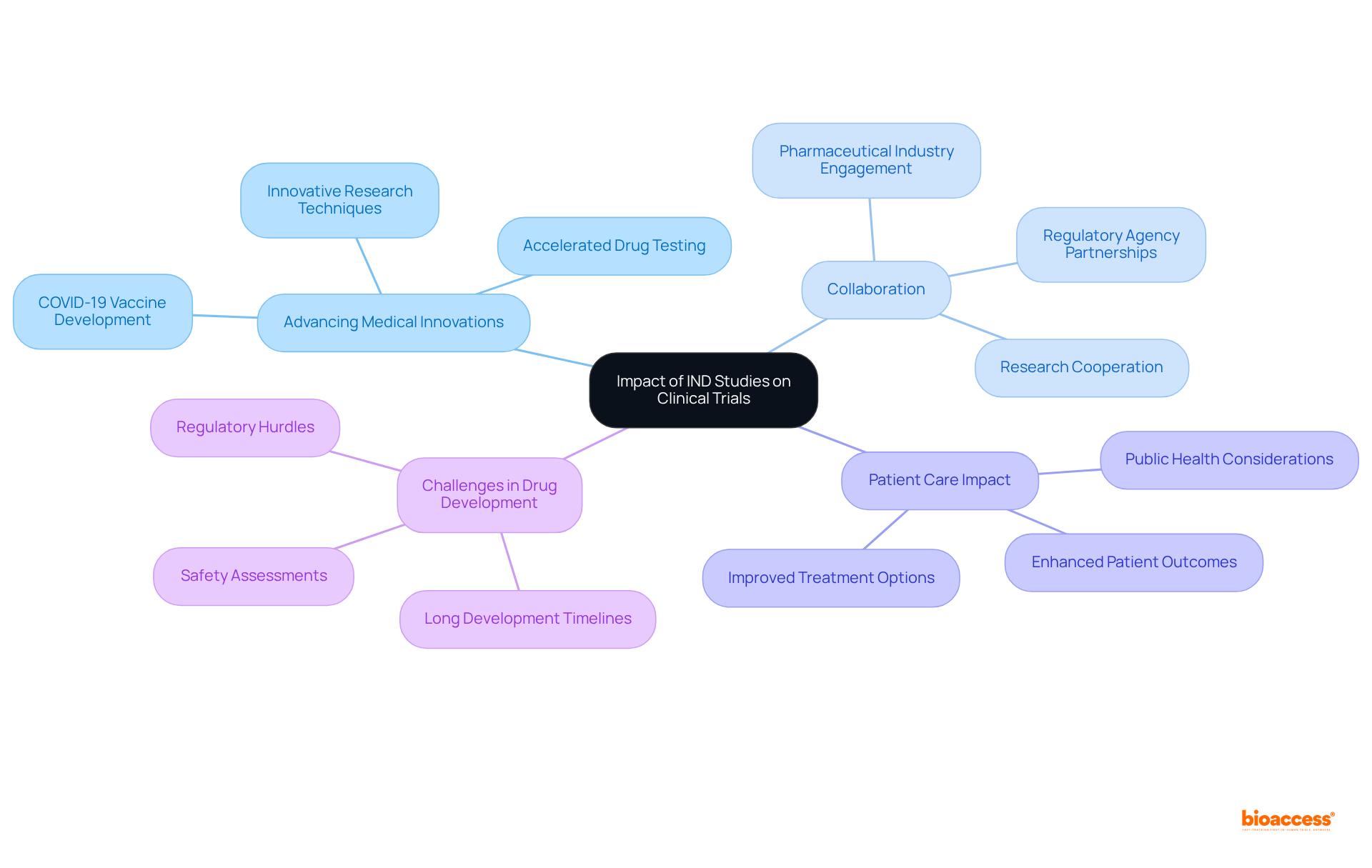
IND research plays a crucial role in advancing medical innovations by enabling the testing of new drugs in human subjects. Successful IND applications lead to clinical trials that can ultimately result in the approval of new therapies, significantly impacting patient care. The rapid development of COVID-19 vaccines exemplifies this, as expedited IND processes allowed for swift testing and deployment of effective treatments. Furthermore, IND investigations foster cooperation among researchers, regulatory agencies, and pharmaceutical firms, creating a robust environment for innovation in healthcare. By rigorously assessing investigational drugs for safety and efficacy, IND research contributes to the overall advancement of medical knowledge and improved patient outcomes.
With over 15 years of expertise, bioaccess® specializes in early-phase research services for the Medtech, Biopharma, and Radiopharma industries, connecting innovative companies with opportunities for conducting research in Latin America, the Balkans, and Australia. This unique value proposition enhances the context of what is IND study, especially considering that the development of a new vaccine typically spans 10 to 15 years. Moreover, the potential public health impact and cost-efficiency of vaccines are vital factors to consider before progressing to trial phases. Animal toxicity data is also essential for determining safe starting doses for human clinical trials, further underscoring the importance of the IND process in the landscape of medical innovation.
An Investigational New Product (IND) study serves as a fundamental building block in the clinical trial landscape, acting as a formal request to the FDA for permission to test new therapies on human subjects. The essence of IND studies lies in their ability to ensure that investigational drugs are safe and scientifically valid before they enter clinical trials. This process is vital for the progression of medical research, as it directly connects laboratory breakthroughs to real-world applications, ultimately enhancing patient care and treatment options.
Throughout this article, key insights into the IND process have been highlighted, including its historical evolution, the rigorous requirements for applications, and its significant impact on advancing medical innovations. Real-world examples demonstrate how successful IND applications have led to the development of groundbreaking therapies, showcasing the importance of these studies in fostering collaboration among researchers, regulatory bodies, and pharmaceutical companies. The efficiency of the IND process, as illustrated by companies like bioaccess®, further emphasizes its role in accelerating the timeline for delivering new treatments to patients.
The implications of IND studies extend far beyond regulatory compliance; they are essential for ensuring patient safety and driving innovation within the medical field. As the landscape of healthcare continues to evolve, understanding the importance of IND studies becomes increasingly critical. Engaging with this process not only supports the development of effective treatments but also cultivates a culture of safety and efficacy in medical research. Embracing the significance of IND studies can lead to transformative advancements in patient outcomes and overall public health.
What is an Investigational New Product (IND) study?
An IND study is a formal petition to the FDA for authorization to administer an investigational substance or biological product to human participants, primarily aimed at assessing the safety of the drug for initial human testing.
What is the primary objective of an IND investigation?
The primary objective of an IND investigation is to ascertain the safety of the investigational drug for initial human testing while providing critical data that supports its scientific validity.
Why are IND investigations important in the clinical trial process?
IND investigations are crucial for advancing medical innovations as they bridge laboratory research and practical application, allowing researchers to rigorously assess the safety and efficacy of novel therapies in human populations.
How does the IND process impact the timeline for delivering new therapies?
Companies like bioaccess® can streamline the IND process, achieving ethical approvals in 4-6 weeks and ensuring participant enrollment is 50% faster than traditional methods, thus hastening the delivery of innovative therapies to patients.
Can you provide examples of successful IND applications?
Yes, EdiGene received IND approval for ET-01, a modified cell therapy for beta-thalassemia using CRISPR-Cas9 technology, and Fate Therapeutics secured IND approval for FT819, a CAR T-cell therapy for acute lymphoblastic leukemia (ALL).
What role do FDA officials play in the IND study process?
FDA officials emphasize the importance of IND studies in ensuring that investigational therapies comply with safety standards before entering clinical trials, which protects participants and fosters innovation in the medical field.