

Software as a Medical Device (SaMD) is software specifically designed for healthcare purposes, functioning independently of hardware medical devices. This innovative technology plays a crucial role in diagnostics, monitoring, and treatment, making it increasingly relevant in today's clinical landscape.
The growing significance of SaMD is underscored by its projected market growth, which reflects a shift towards digital solutions in healthcare. Evolving regulatory frameworks are also pivotal, ensuring safety and efficacy while fostering innovation. This dual focus not only highlights the challenges faced in SaMD development but also presents numerous opportunities for advancement.
As we navigate this dynamic Medtech landscape, it is essential to recognize the importance of collaboration among stakeholders. By working together, we can address key challenges and leverage the potential of SaMD to enhance patient care and outcomes.
Understanding the role of Software as a Medical Device (SaMD) is increasingly vital as healthcare evolves. This innovative category of software operates independently of hardware, transforming patient care by providing tools that autonomously diagnose, monitor, and treat medical conditions. With the SaMD market projected to soar to $8.2 billion by 2027, stakeholders face significant challenges in navigating the complex regulatory landscape. How can they harness the potential of SaMD while ensuring compliance and safety in an ever-changing medical environment?
The importance of SaMD cannot be overstated. As healthcare continues to advance, the ability to leverage technology for improved patient outcomes is paramount. By understanding the intricacies of SaMD, stakeholders can position themselves to not only comply with regulations but also to lead in innovation. This article will explore the Medtech landscape and the role of bioaccess in addressing key challenges, emphasizing the need for collaboration and strategic action.
To understand what is samd, it is important to know that Software as a Medical Device (SaMD) encompasses software designed for healthcare purposes that operates independently of hardware medical devices. This includes applications capable of diagnosing, preventing, monitoring, or treating medical conditions autonomously. For example, consider a mobile application that evaluates a patient's health information to suggest treatment options; this is a prime illustration of what is samd. The International Medical Device Regulators Forum (IMDRF) defines such software, underscoring its critical role in the evolving medical landscape.
As the market for SaMD applications is projected to grow significantly, reaching an estimated $8.2 billion by 2027, understanding what is samd becomes crucial in enhancing patient care and streamlining medical service delivery. In this context, bioaccess® offers comprehensive clinical trial management services, including:
These services are essential for Medtech, Biopharma, and Radiopharma startups navigating the complexities of regulatory approval and trial setup for applications related to what is samd.
By leveraging bioaccess's expertise, companies can expedite their clinical trials, ensuring adherence to local regulations and increasing the chances of successful outcomes. Collaboration in this space is vital, and the next steps involve engaging with bioaccess to harness their knowledge and resources effectively.

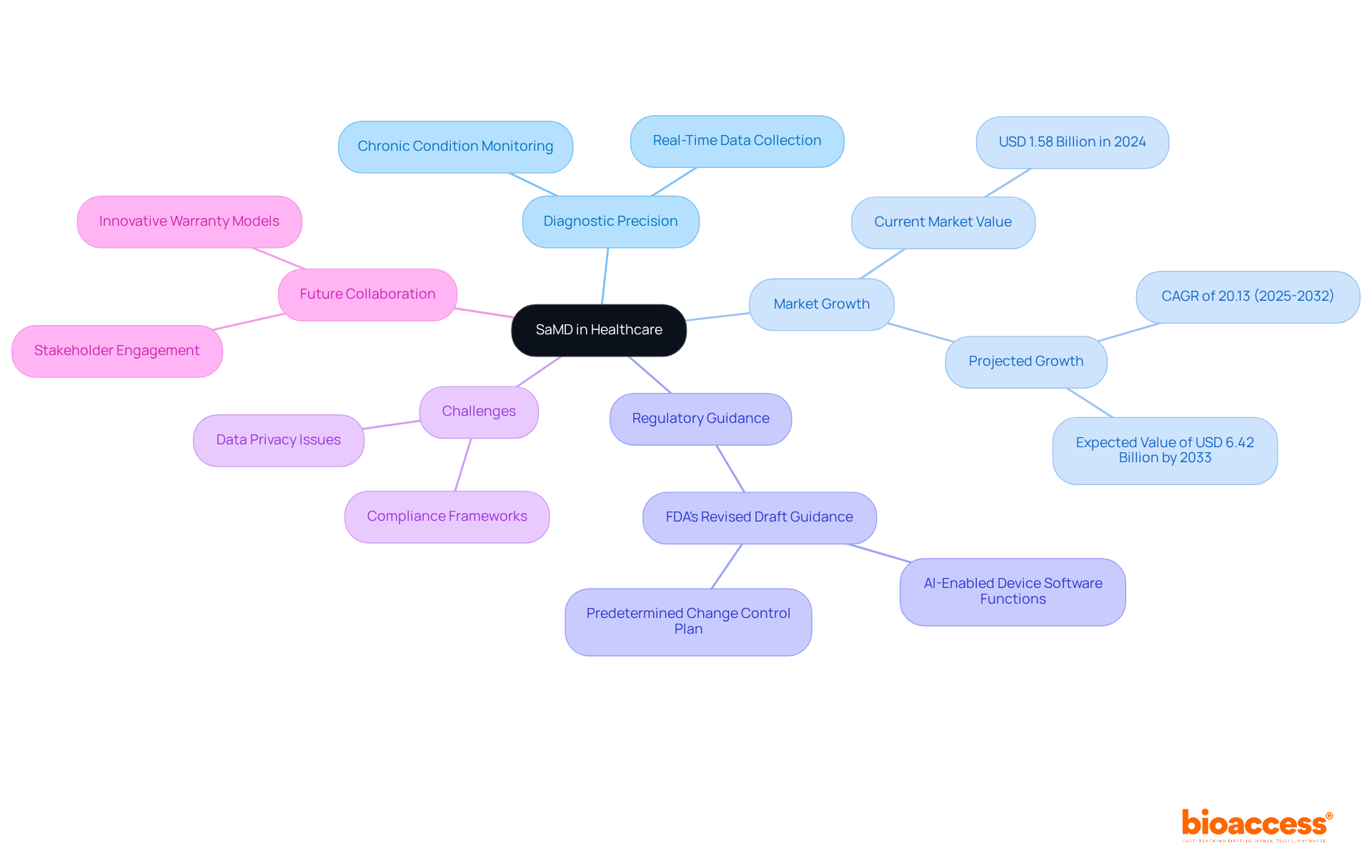
What is SaMD plays a crucial role in contemporary medical practice by enhancing diagnostic precision, improving patient observation, and facilitating individualized treatment strategies. As telemedicine and digital health solutions rise, it’s important to understand what is SaMD and its growing significance. For example, applications that monitor chronic conditions like diabetes or heart disease enable real-time data collection and analysis, allowing healthcare providers to make informed decisions swiftly.
The integration of AI and machine learning into SaMD platforms is driving significant advancements, which illustrates what is SaMD and enhances the capabilities of these solutions. The SaMD market, valued at USD 1.58 billion in 2024, is projected to grow at a CAGR of 20.13% through 2032, with expectations to reach USD 6.42 billion by the end of 2033. This underscores the increasing demand for innovative solutions in healthcare.
The FDA's revised draft guidance on AI-Enabled Device Software Functions, issued in May 2025, highlights the evolution of the landscape, ensuring safety and efficacy while promoting innovation. However, challenges such as data privacy issues and strict compliance frameworks remain vital matters that the SaMD market must navigate. Additionally, various warranty models, including Performance-Based and Outcome-Based warranties, are gaining traction, aligning incentives among stakeholders and supporting value-based care initiatives.
Incorporating what is SaMD into clinical workflows not only streamlines processes but also empowers patients to take an active role in managing their health. As we look to the future, collaboration among stakeholders will be essential in overcoming challenges and harnessing the full potential of these innovative solutions.
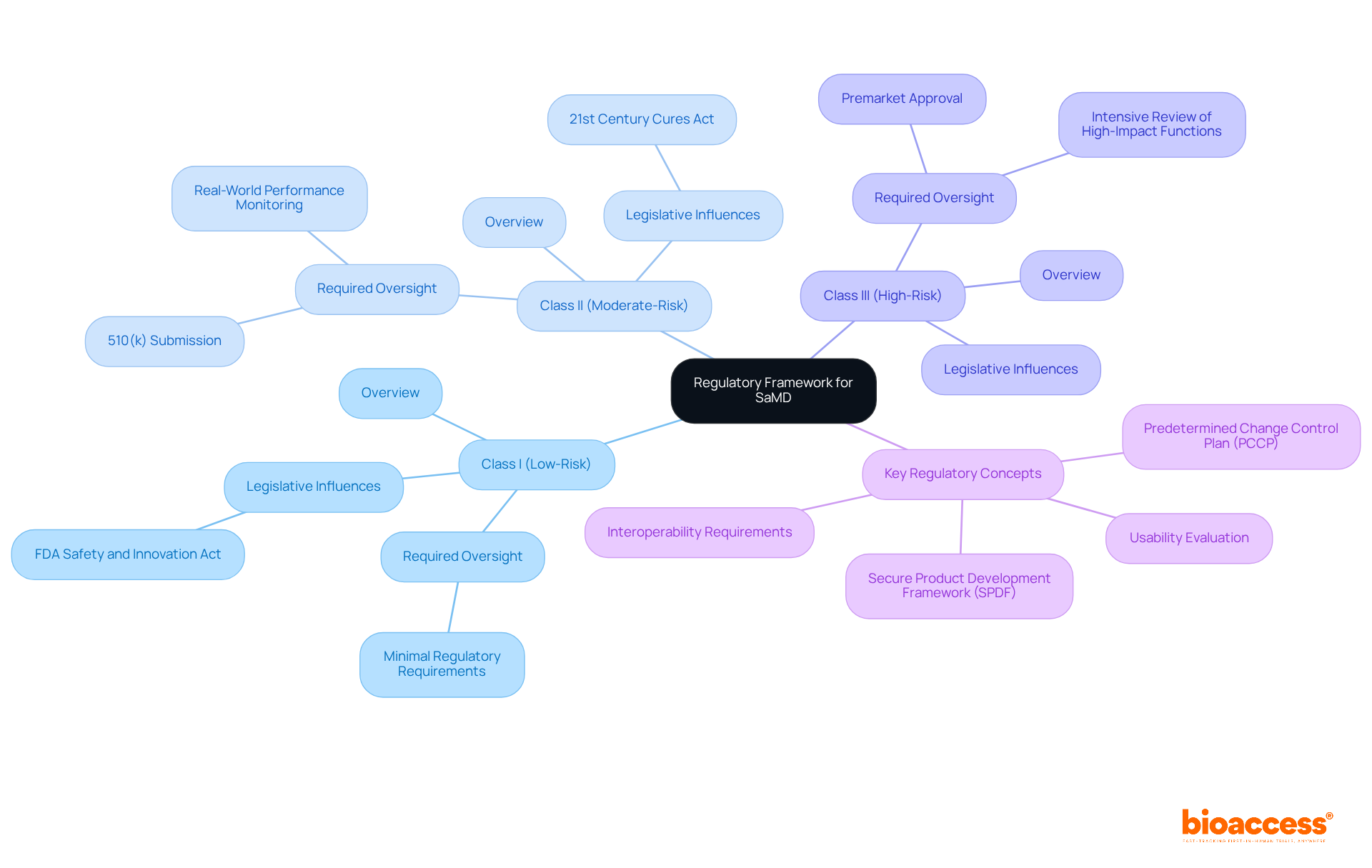
The framework for Software as a Medical Device is shaped by recommendations from key organizations, notably the FDA in the United States and the European Medicines Agency in Europe. The FDA classifies software as a medical device into three risk categories:
Each category dictates the level of oversight required. By 2025, the FDA's classification system reflects a nuanced understanding of software as a medical device, ensuring that products are evaluated based on their specific functions and potential impacts on patient health.
In recent years, the FDA has revised its oversight strategy, influenced by legislative initiatives like the FDA Safety and Innovation Act and the 21st Century Cures Act. These changes have led to the adoption of flexible oversight frameworks, which include real-world performance assessments and adaptable change protocols. Understanding what is samd is essential for navigating the complexities of software as a medical device regulation.
Experts such as Ana Criado, Director of Regulatory Affairs and a professor in biomedical engineering, play a pivotal role in this evolving landscape. With her extensive experience in regulatory affairs and health economics, along with her consultancy work for global companies, she significantly influences the understanding and application of these regulations in Colombia and beyond. Similarly, Katherine Ruiz, an expert in Regulatory Affairs for Medical Devices and In Vitro Diagnostics in Colombia, is instrumental in ensuring compliance and advocating for best practices in the field.
The International Medical Device Regulators Forum (IMDRF) has also been vital in harmonizing regulations across different jurisdictions, fostering global consistency in the oversight of software as a medical device. Adhering to these regulations is crucial, as products in this category must undergo rigorous testing and validation to ensure their safety and effectiveness before entering the market. This comprehensive governance structure not only safeguards patient well-being but also facilitates the successful marketing of innovative software as a medical device solutions.
Creating Software as a Medical Device (SaMD) involves understanding what is SaMD and navigating a landscape marked by intricate compliance demands, particularly in Colombia, where INVIMA (Colombia National Food and Drug Surveillance Institute) plays a crucial role. As a Level 4 health authority recognized by the Pan American Health Organization/World Health Organization, INVIMA oversees the marketing and manufacturing of health products, including medical devices, through its Directorate for Medical Devices and other Technologies. This oversight ensures adherence to safety, efficacy, and quality standards, yet it also introduces complexities that developers must confront, including detailed approval procedures.
Robust data security and interoperability with existing healthcare systems are essential. The rapid pace of technological advancement often outstrips regulatory frameworks, creating uncertainty for developers. However, these challenges also present opportunities for innovation. For example, the integration of artificial intelligence (AI) and machine learning can significantly enhance the capabilities of software as a medical device, leading to improved diagnostic accuracy and personalized treatment options. Organizations that effectively navigate these challenges while leveraging emerging technologies are well-positioned to thrive in the evolving medical landscape. The adoption of AI in software for medical devices is on the rise, with many organizations harnessing these advancements to enhance diagnostics and optimize patient outcomes. By prioritizing innovation alongside regulatory compliance, developers can fully realize what is SaMD, which ultimately contributes to superior healthcare solutions.

Understanding Software as a Medical Device (SaMD) is crucial in today’s healthcare landscape, where technology increasingly shapes patient care and treatment. SaMD refers to software intended for medical purposes, functioning independently of hardware devices, and it holds the potential to revolutionize healthcare delivery through improved diagnostics and tailored treatment strategies.
This article has delved into the definition of SaMD, its rising importance in healthcare, and the regulatory frameworks that govern its development. Key insights include:
Moreover, the evolving regulatory landscape for SaMD, particularly from organizations like the FDA and IMDRF, underscores the necessity for stringent oversight to ensure safety and efficacy.
As the SaMD market continues to grow, it brings both challenges and opportunities for innovation. Stakeholders must adeptly navigate complex regulatory environments while embracing technological advancements to improve healthcare outcomes. Engaging with experts and utilizing available resources can facilitate the successful development and implementation of SaMD solutions, ultimately leading to enhanced patient care and a more efficient healthcare system.
What is Software as a Medical Device (SaMD)?
Software as a Medical Device (SaMD) refers to software designed for healthcare purposes that operates independently of hardware medical devices. It includes applications capable of diagnosing, preventing, monitoring, or treating medical conditions autonomously.
Can you provide an example of SaMD?
An example of SaMD is a mobile application that evaluates a patient's health information to suggest treatment options.
What organization defines SaMD and its importance?
The International Medical Device Regulators Forum (IMDRF) defines SaMD, highlighting its critical role in the evolving medical landscape.
What is the projected market growth for SaMD applications?
The market for SaMD applications is projected to grow significantly, reaching an estimated $8.2 billion by 2027.
What services does bioaccess® offer related to SaMD?
Bioaccess® offers comprehensive clinical trial management services, including feasibility studies, site selection, compliance reviews, and project management.
Why are bioaccess's services important for startups in the Medtech, Biopharma, and Radiopharma sectors?
These services are essential for startups navigating the complexities of regulatory approval and trial setup for applications related to SaMD, helping to expedite clinical trials and ensure adherence to local regulations.
How can companies benefit from collaborating with bioaccess?
By leveraging bioaccess's expertise, companies can increase the chances of successful outcomes in their clinical trials and streamline their processes in the medical device landscape.