


In clinical research, the control group serves a crucial role as a benchmark for comparison. This allows researchers to isolate the effects of an experimental intervention and accurately evaluate its true efficacy. The article underscores this importance by detailing how control groups, such as placebo and no-treatment groups, effectively mitigate biases and confounding factors. By doing so, they ensure the reliability and validity of study outcomes, which is essential for advancing medical knowledge and improving patient care.
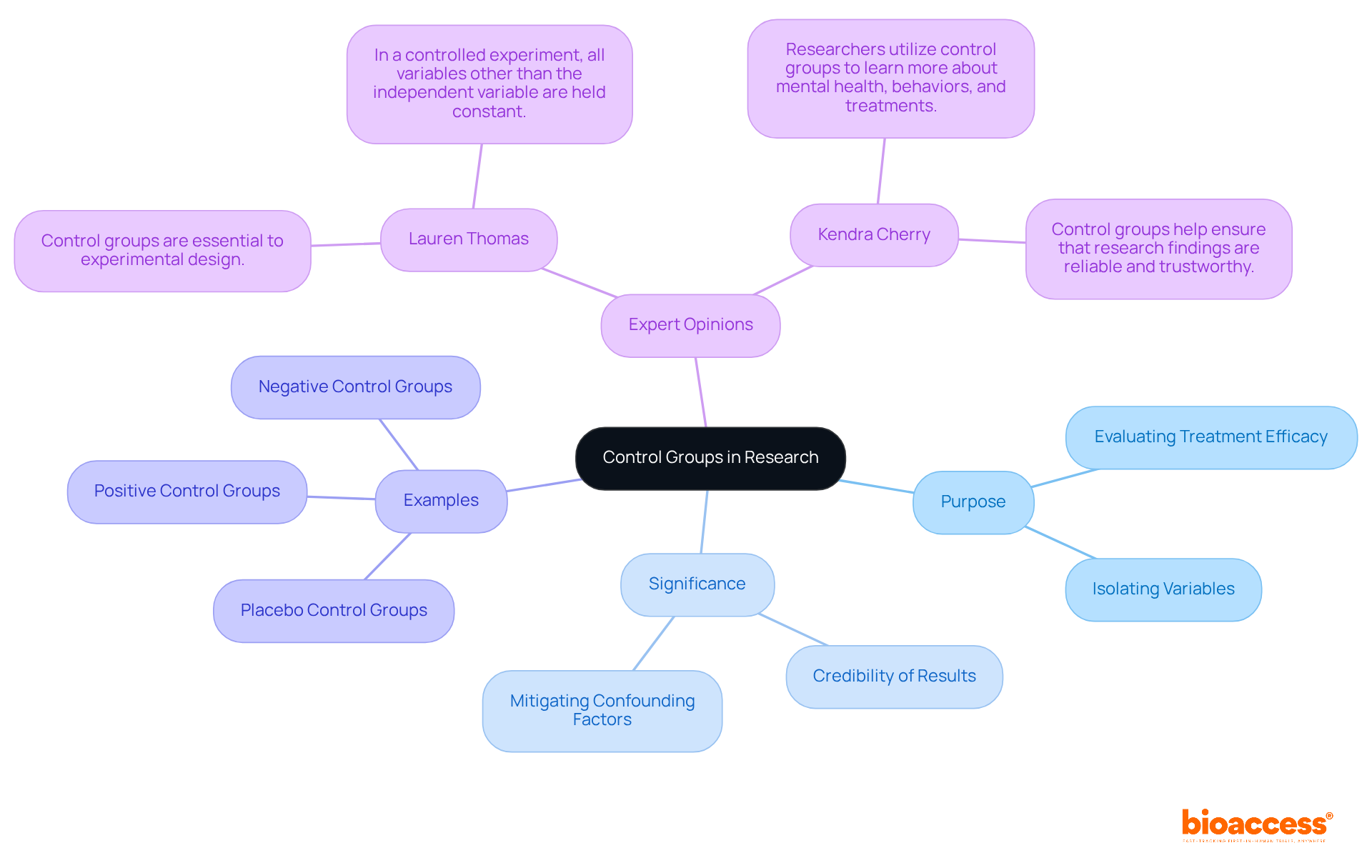
Control groups are fundamental to experimental research, acting as a crucial reference point that enables scientists to identify the true effects of an intervention. By comparing outcomes between a control group and an experimental group, researchers can effectively isolate the impact of the independent variable, thereby ensuring that their findings are both valid and reliable.
Yet, a pivotal question arises: what occurs when these essential components are disregarded? Recognizing the purpose of control groups not only bolsters the credibility of research but also unveils the complex interplay between treatment effects and external influences. This understanding is vital for anyone involved in scientific inquiry.
Control sets represent a fundamental aspect of experimental research, as they help clarify what is the purpose of a control group by serving as an essential reference point for evaluating the impacts of an experimental intervention. Typically, the reference set does not undergo the procedure or intervention being assessed, allowing researchers to observe results under normal conditions. This comparison is crucial for evaluating treatment efficacy and understanding what is the purpose of a control group in isolating the effects of the independent variable on the dependent variable. For example, in clinical trials evaluating new medications, the reference set often receives a placebo, while the experimental set is administered the actual drug. This design empowers researchers to draw valid conclusions regarding the medication's effectiveness.
The significance of experimental sets extends beyond mere comparison; they enhance the credibility of study results. By ensuring that all other variables remain constant, experimental sets mitigate the influence of confounding factors, thereby bolstering the reliability of the findings. In fact, approximately 70% of clinical trials utilize comparison sets, underscoring their importance in establishing causality and dependability in research outcomes.
Expert opinions highlight that what is the purpose of a control group is essential not only in clinical studies but also across various research domains. They facilitate a clearer understanding of treatment effects, enabling researchers to directly attribute changes in the dependent variable to the independent variable. This rigorous methodology is fundamental in advancing scientific knowledge and ensuring that findings are both valid and reproducible.
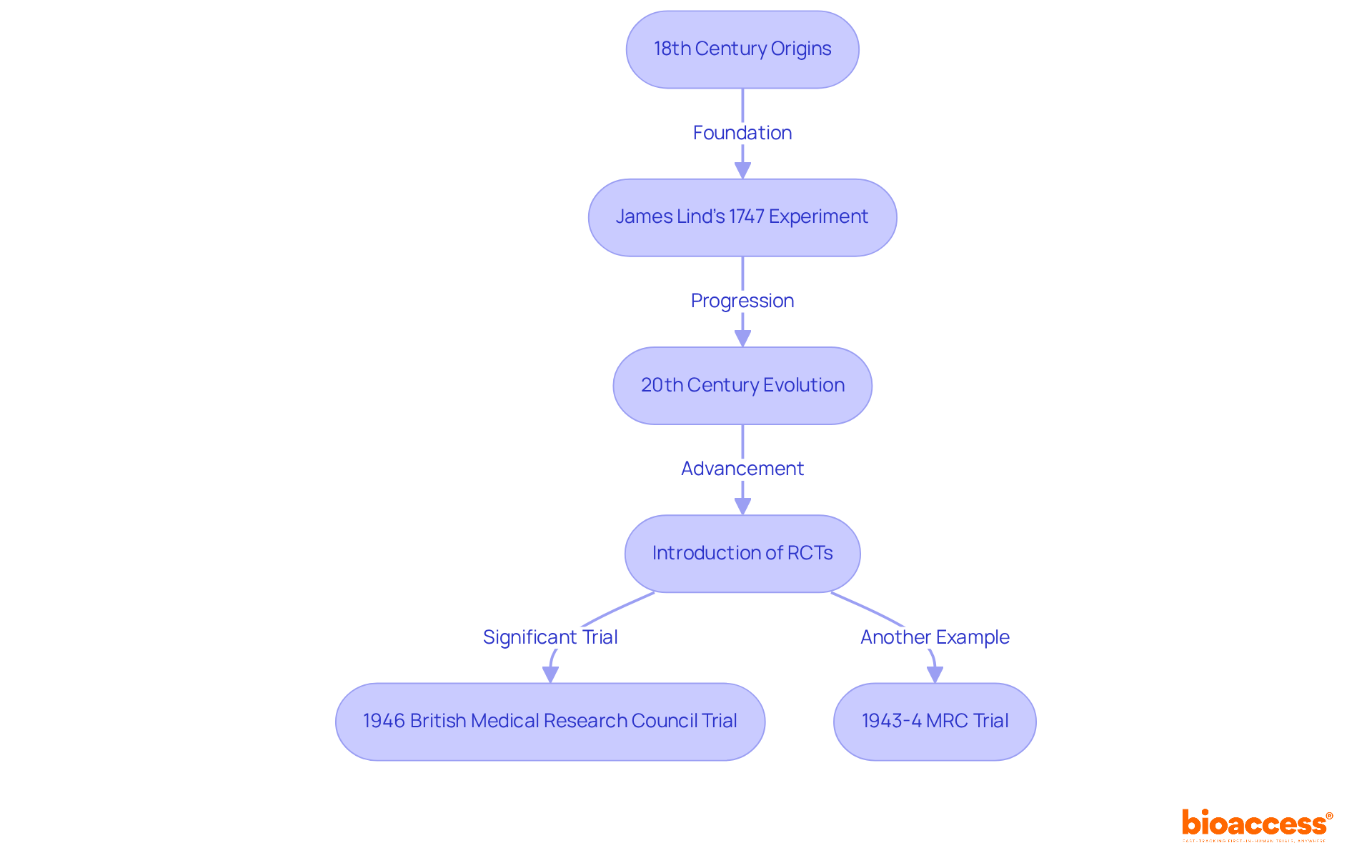
The concept of experimental comparison sets is fundamental in scientific testing, with origins traceable to the 18th century. A pivotal example is Scottish physician James Lind's experiment in 1747, where he explored the effects of citrus fruits on sailors suffering from scurvy. Lind's methodology involved contrasting groups of sailors who received various dietary supplements, successfully establishing a reference group that did not receive citrus. This initial application of a testing cohort laid the groundwork for future clinical trials.
As clinical research evolved, particularly in the 20th century, the approach to comparison sets underwent significant enhancement. The advent of randomized controlled trials (RCTs) marked a transformative shift, instituting a more rigorous framework for hypothesis testing. RCTs ensure that comparison participants are randomly allocated, reducing bias and confounding factors, thereby enhancing the validity of outcomes. Notably, the 1946 British Medical Research Council trial of streptomycin for tuberculosis was the first to integrate randomization, meticulous recruitment, and careful specification of interventions, setting a precedent for the systematic use of comparison cohorts in clinical trials.
Historical examples further underscore the importance of experimental cohorts. The 1946 trial not only confirmed the effectiveness of streptomycin but also shaped methodologies for decades. Additionally, the 1943-4 MRC trial investigating patulin for the common cold serves as another relevant illustration of evolving comparative methodology.
In summary, the advancement of experimental methodology reflects an ongoing commitment to enhancing the integrity and reliability of clinical studies, ensuring that results significantly contribute to medical progress. Understanding what is the purpose of a control group is crucial for grasping the concept of the placebo effect, as it emphasizes the importance of distinguishing the effects of active interventions from those perceived by patients. This evolution illustrates a growing awareness of the necessity for scientific rigor and ethical considerations in investigative practices.
Control groups play a crucial role in research, and one might ask, what is the purpose of a control group, as they are categorized into several types, each serving a unique role.
Placebo Control Group: Participants receive a placebo treatment, enabling researchers to evaluate the psychological effects of treatment. This design is vital for differentiating the true effectiveness of a new intervention from the placebo effect, where participants may experience changes solely due to their belief in the remedy.
No-Treatment Control Set: This set does not receive any intervention, providing a baseline for comparison. It allows researchers to assess the natural progression of conditions without any intervention influence, thereby enhancing the internal validity of the study.
Active Control Team: Participants in this team receive an existing treatment, allowing researchers to compare the new treatment's efficacy against a standard. This approach is particularly valuable in clinical trials, as it establishes a benchmark for evaluating the effectiveness of innovative therapies.
Historical Comparison Group: In this design, data from prior studies serve as a reference. This method is beneficial when a new study cannot incorporate a conventional reference set, enabling researchers to make comparisons using historical data while acknowledging possible variations in situations.
Each category of comparison significantly impacts the validity and reliability of research findings, leading to the question of what is the purpose of a control group in influencing how results are interpreted. For instance, in Medtech research, the use of placebo comparison cohorts is essential for establishing the actual effectiveness of new medical devices or therapies, as it aids in separating the effect of the intervention from psychological factors. As noted in the literature, "Control sets are essential for assessing the effectiveness of new treatments." Furthermore, challenges such as ethical considerations and practical limitations must be addressed when implementing control sets. By understanding these variations, scholars can design studies that effectively tackle their specific inquiries and ethical considerations.

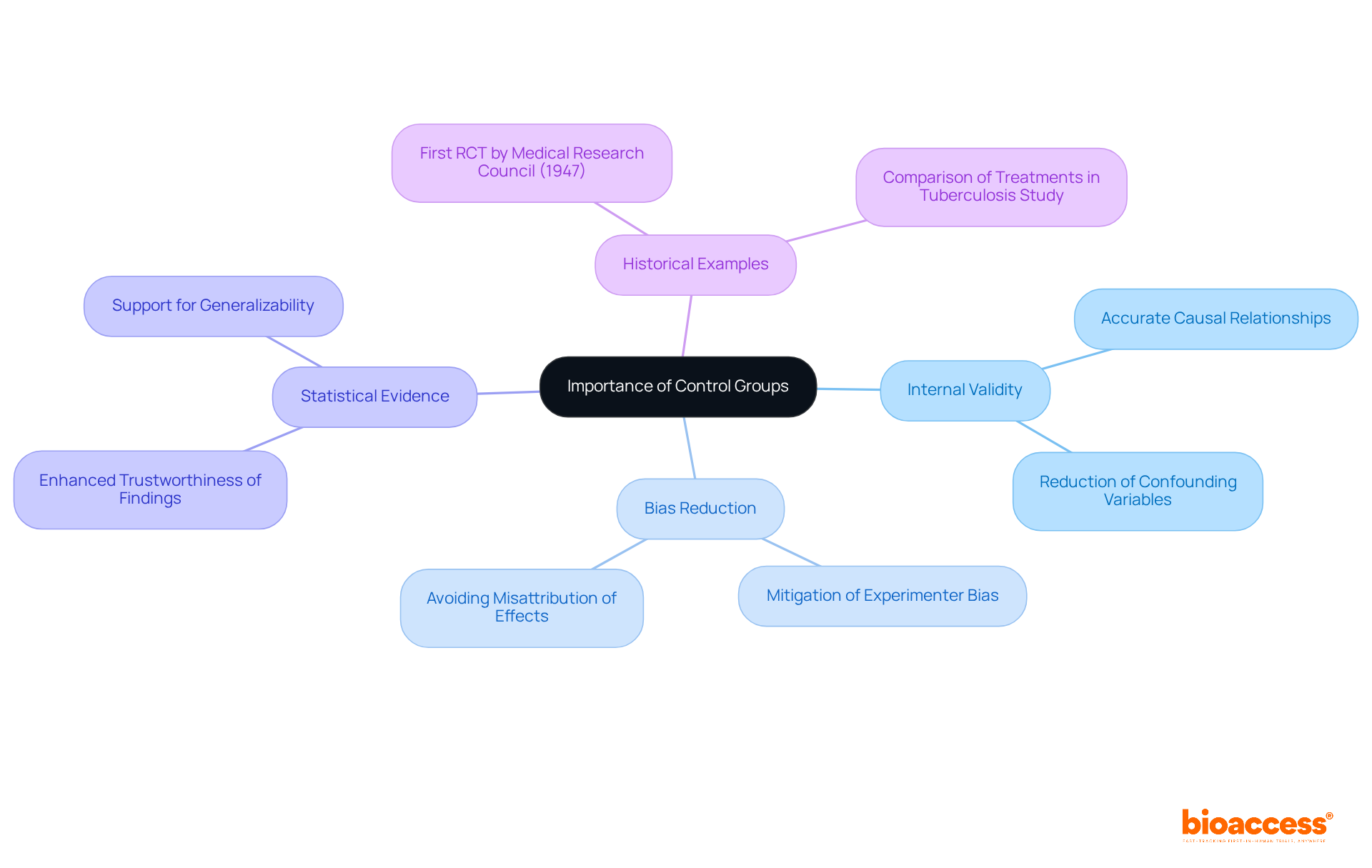
Control cohorts are essential for preserving the internal validity of research studies, as they help answer the question of what is the purpose of a control group by serving as a benchmark for comparison. They allow researchers to determine what is the purpose of a control group in assessing whether observed effects arise from the intervention itself or from confounding factors.
In the absence of a reference set, precisely evaluating the genuine effect of an intervention becomes challenging, frequently resulting in biases in the outcomes. For instance, in clinical studies lacking a reference cohort, researchers may mistakenly ascribe enhancements in patient results exclusively to the new intervention, overlooking other factors like natural recovery or placebo effects.
Statistics indicate that research devoid of comparison samples is considerably more susceptible to biases, which can skew results and mislead interpretations. By including comparison sets, researchers can effectively mitigate these biases, ensuring their conclusions are grounded in trustworthy evidence.
Historical examples illustrate this point; the first randomized trial (RCT) conducted by the UK's Medical Research Council in 1947 demonstrated how comparison sets could isolate treatment effects, ultimately advancing the field of clinical research.
Therefore, to understand what is the purpose of a control group, we see that they are not merely a methodological requirement but a cornerstone for drawing accurate conclusions and fostering scientific progress.
Control groups are a vital component of research methodology, serving as benchmarks that enable researchers to isolate the effects of interventions. By providing a point of comparison, these groups enhance the validity of findings, ensuring that observed changes can be attributed to the independent variable rather than external factors. This fundamental principle highlights the essential role of control groups across various research domains, from clinical trials to social sciences.
The multifaceted roles of control groups have been explored, including their historical evolution and the various types that exist, such as:
Each type plays a unique role in safeguarding the integrity of research outcomes, with randomized controlled trials exemplifying how these methodologies can mitigate bias and improve result reliability. The historical context underscores the advancement in the understanding and application of control groups, contributing to the scientific rigor necessary for credible research.
Recognizing the significance of control groups is crucial for anyone engaged in research. By employing these methodologies, researchers can draw more accurate conclusions, foster scientific advancement, and contribute to evidence-based practices. The commitment to utilizing control groups not only enhances the reliability of studies but also upholds the ethical standards of scientific inquiry, paving the way for future innovations and discoveries.
What is the purpose of a control group in research?
The purpose of a control group is to serve as a reference point for evaluating the impacts of an experimental intervention, allowing researchers to observe results under normal conditions without the intervention.
How do control groups help in evaluating treatment efficacy?
Control groups help isolate the effects of the independent variable on the dependent variable, enabling researchers to draw valid conclusions about the effectiveness of a treatment or intervention.
What is a common practice in clinical trials regarding control groups?
In clinical trials, the control group often receives a placebo, while the experimental group receives the actual treatment, such as a new medication, to assess the treatment's effectiveness.
Why are control groups important for the credibility of study results?
Control groups enhance the credibility of study results by ensuring that all other variables remain constant, which mitigates the influence of confounding factors and bolsters the reliability of the findings.
How prevalent are control groups in clinical trials?
Approximately 70% of clinical trials utilize control groups, highlighting their importance in establishing causality and dependability in research outcomes.
In what other research domains are control groups significant?
Control groups are significant not only in clinical studies but also across various research domains, as they facilitate a clearer understanding of treatment effects and help attribute changes in the dependent variable directly to the independent variable.
What is the overall impact of using control groups in research?
The use of control groups is fundamental in advancing scientific knowledge, ensuring that findings are both valid and reproducible.