


Accuracy and precision are paramount for achieving success in clinical research, as they underpin the reliability of trial outcomes and the safety of patients. This article underscores the significance of upholding high standards in these areas, which not only prevents misleading conclusions but also mitigates regulatory setbacks. Such diligence is essential for safeguarding patient welfare and bolstering the credibility of medical findings. By prioritizing accuracy and precision, researchers can ensure that their work contributes meaningfully to the advancement of medical knowledge and patient care.
In the realm of clinical research, accuracy and precision serve as foundational pillars that uphold the integrity of medical studies. Their significance transcends mere data collection; they have a direct impact on patient safety, treatment efficacy, and the reliability of research findings.
With nearly 30% of medical studies failing due to inaccuracies, a pressing question emerges: how can researchers guarantee that their data is both accurate and precise?
Delving into this critical intersection will unveil strategies that not only enhance the quality of clinical trials but also protect the trust placed in medical research.
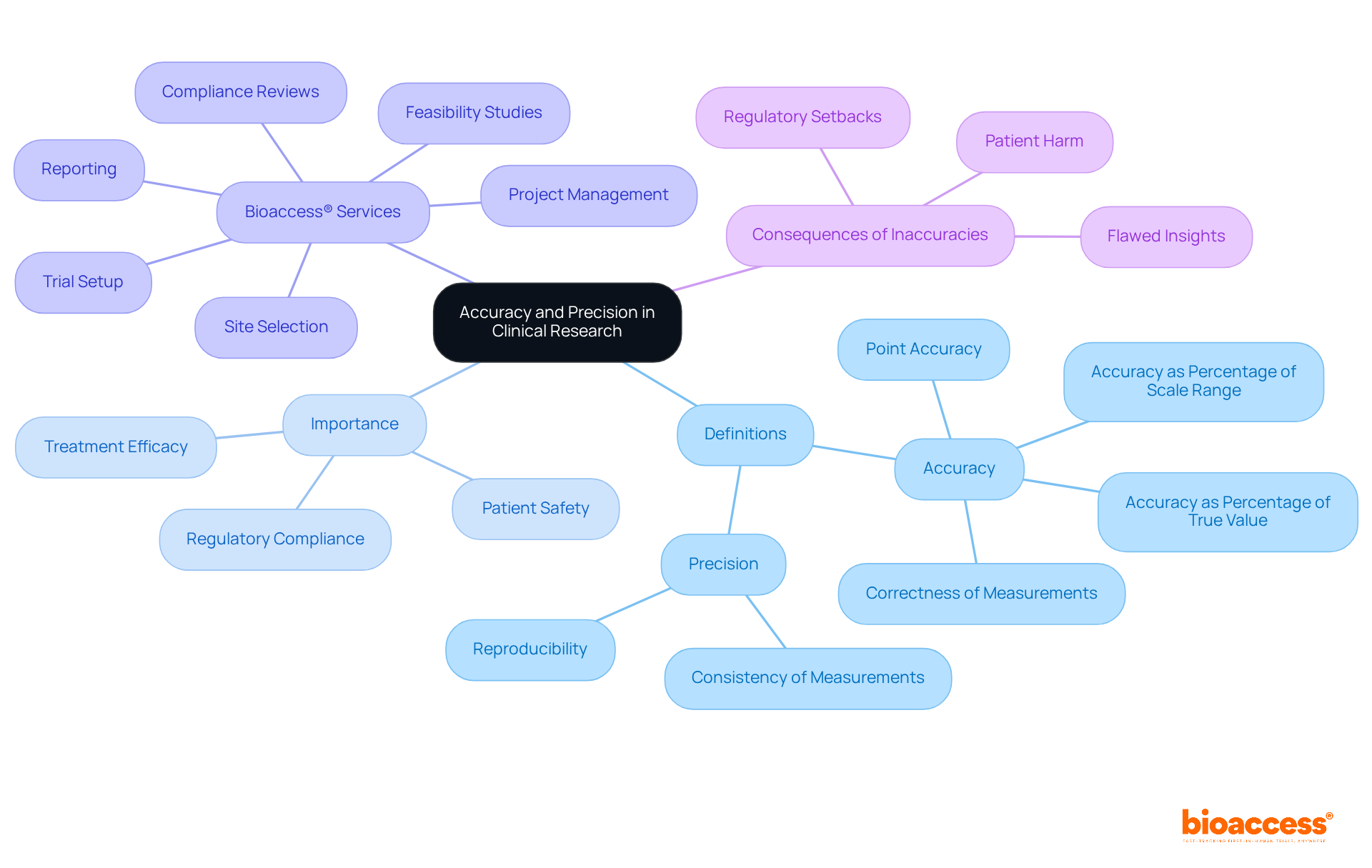
In medical research, accuracy is defined as the degree to which a measured value aligns with a known standard, while consistency refers to the reliability of repeated measurements. For example, in a study evaluating blood pressure, accuracy indicates that recorded readings closely match the true blood pressure of subjects, whereas consistency suggests that repeated measurements yield similar results. This distinction is crucial; as noted, 'Measurements can be both accurate and precise, accurate but not precise, precise but not accurate, or neither.'
Understanding these definitions is vital for evaluating the reliability of medical data and outcomes, as accuracy and precision define this reliability. Both accuracy and precision define the essential criteria for validating research findings and ensuring their applicability to real-world scenarios, ultimately influencing patient safety and treatment efficacy.
As emphasized by bioaccess®, upholding these principles throughout the research process is critical, particularly in specialized studies across diverse regions. Bioaccess® offers comprehensive trial management services, including:
All aimed at maintaining these standards. Inaccuracies can lead to flawed insights, regulatory setbacks, and even harm to patients, making it imperative that accuracy and precision define clinical research.
Moreover, statistics reveal that error rates for single-information entry can range from 0.29% to 2.784 mistakes per 10,000 fields, underscoring the need for rigorous management practices. The case study titled 'The Importance of Accuracy and Precision Define in Clinical Trials' further illustrates how these concepts are essential for ensuring information integrity and supporting regulatory compliance.
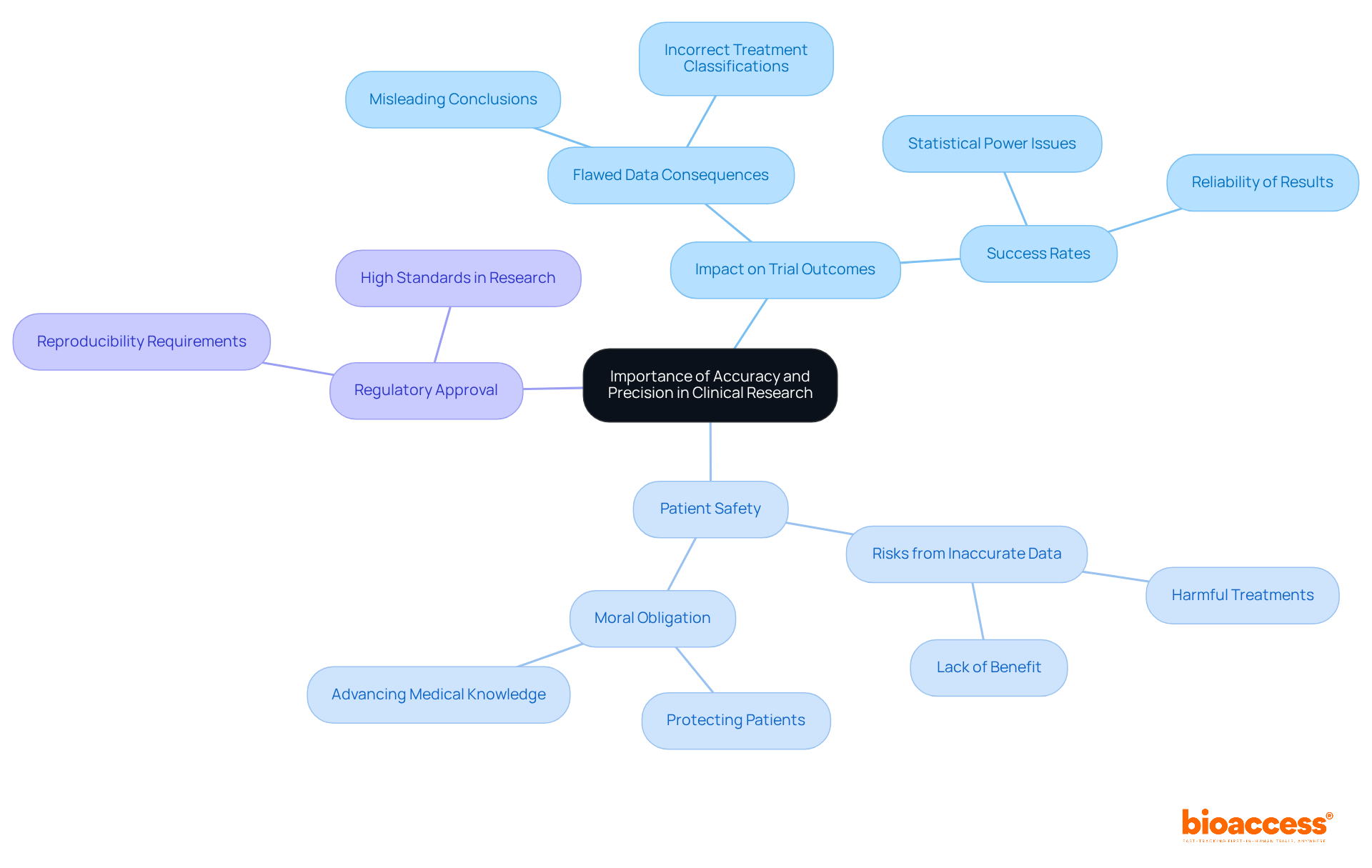
Accuracy and precision define essential pillars of clinical research, significantly impacting the validity of trial outcomes. Flawed information can lead to misleading conclusions regarding a treatment's efficacy, posing serious risks to patient safety. For example, inaccurate data may result in a medication being incorrectly classified as effective, causing patients to receive treatments that provide no benefit or, in some instances, inflict harm.
Accuracy and precision define the importance of ensuring that results are reproducible, which is a critical factor for regulatory approval and acceptance within the medical community. The high standards that accuracy and precision define are not merely regulatory requirements; they represent a moral obligation to protect patients and foster advancements in medical knowledge.
Furthermore, research has shown that a lack of accuracy can significantly diminish the success rates of medical studies, as variable outcomes hinder the ability to reach reliable conclusions. Expert insights underscore that maintaining strict standards in information gathering and analysis is vital for enhancing patient safety and ensuring that research positively contributes to healthcare outcomes.
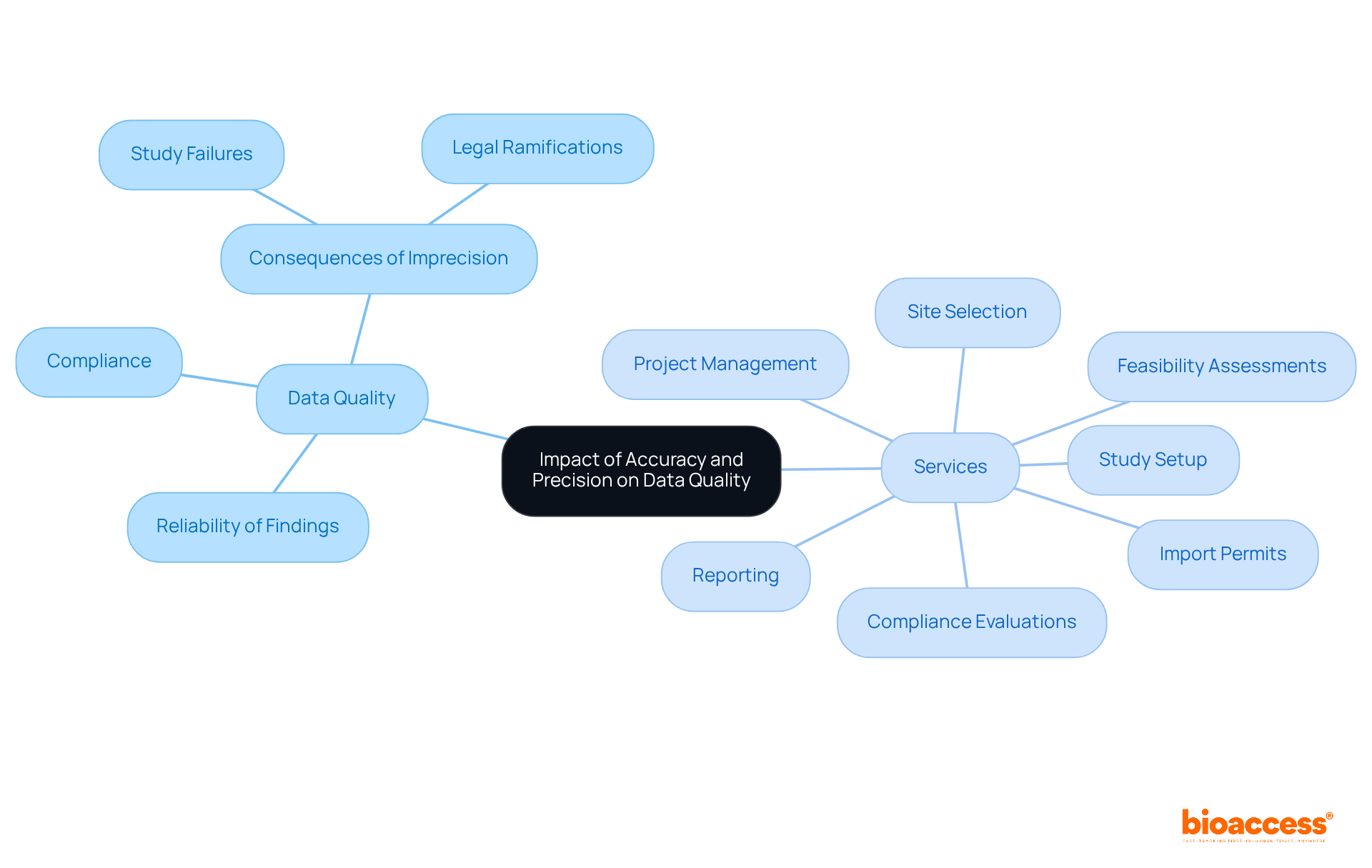
Accuracy and precision define the quality of information in research trials, directly influencing adherence to regulatory standards. Regulatory agencies, including the FDA, mandate that trial information be accurate and precise because accuracy and precision define the reliability of findings. Notably, approximately 30% of medical studies fail due to information-related issues, underscoring the critical importance of meticulous information management.
At bioaccess, our comprehensive clinical study management services encompass:
All meticulously designed to enhance information integrity. Implementing electronic information capture (EDC) can diminish entry errors by up to 50%, thereby further elevating quality. Faulty or imprecise information can result in non-compliance, leading to significant delays in approval processes or outright rejection of studies, along with potential legal ramifications such as fines or suspension of trial approvals.
High-quality information not only bolsters the credibility of research findings but also cultivates trust among stakeholders, including patients, healthcare providers, and regulatory agencies. Comprehensive information sets are indispensable for thorough examination and to reinforce the reliability of research outcomes. The management team at bioaccess plays a pivotal role in ensuring compliance with protocols and the accurate recording of information during studies, conducting regular audits to maintain quality.
Therefore, prioritizing accuracy and precision define the essential steps for preserving data integrity and ensuring compliance with regulatory standards, ultimately facilitating the advancement of medical knowledge and patient safety.
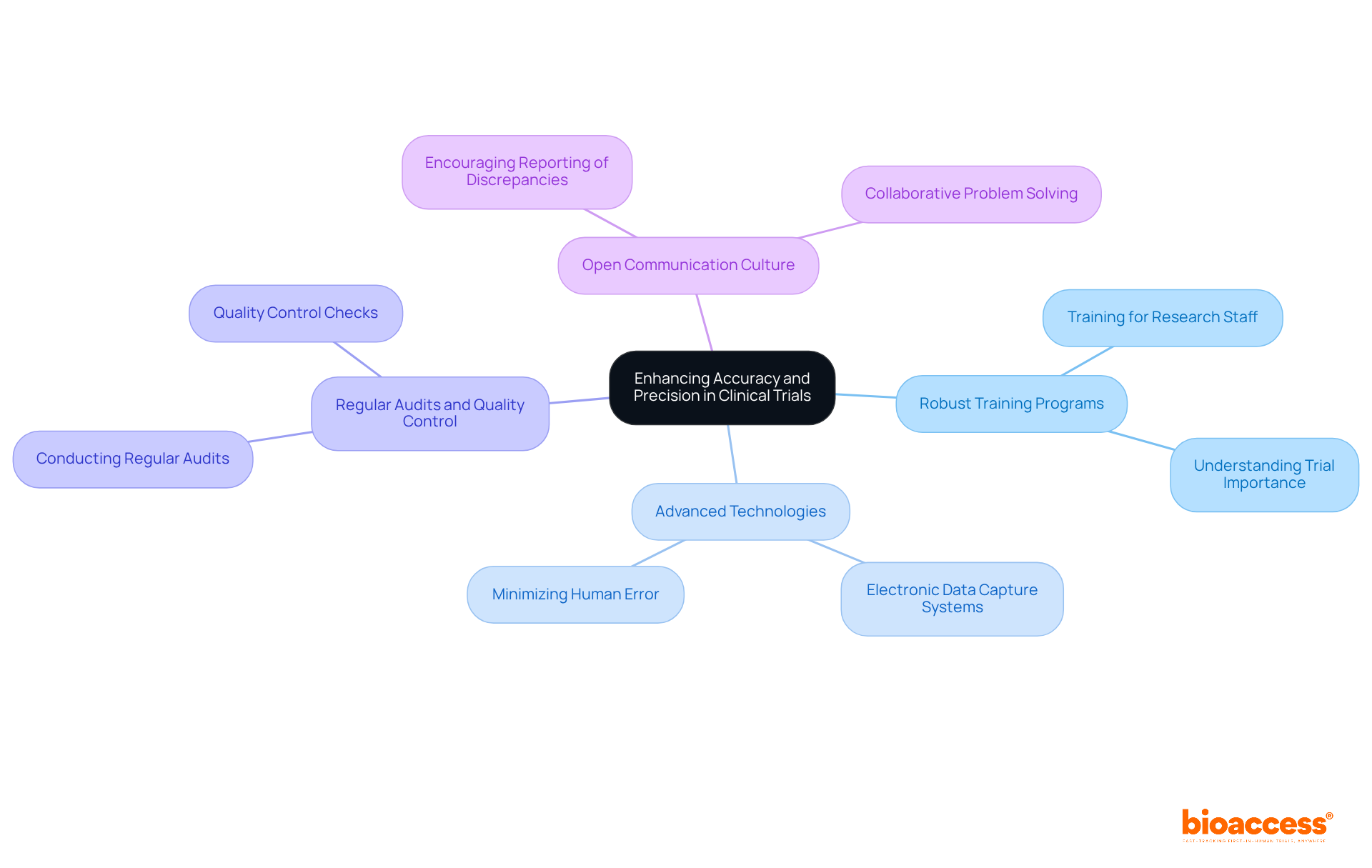
Improving accuracy in clinical trials is essential and can be effectively achieved through various strategies, particularly by leveraging the extensive clinical trial management services offered by bioaccess®.
The implementation of robust training programs for research staff is crucial; it ensures that all participants grasp the significance of these concepts and the methods to realize them. With over 20 years of Medtech expertise, bioaccess® specializes in Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF), all of which accuracy and precision define as vital for upholding high standards.
The utilization of advanced technologies, such as electronic data capture systems, minimizes human error and enhances data consistency.
Conducting regular audits and quality control checks throughout the trial process is imperative to swiftly identify and rectify inaccuracies.
Fostering a culture of open communication among team members is essential; it encourages the reporting of discrepancies, which can then be addressed collaboratively.
By adopting these strategies and partnering with bioaccess®, clinical researchers can significantly enhance what accuracy and precision define in their studies, ultimately leading to more reliable outcomes.
Accuracy and precision are foundational elements in clinical research, fundamentally shaping the success and integrity of medical studies. Their significance is paramount, as they directly influence the validity of outcomes and the safety of patient care. Ensuring that data aligns with established standards and remains consistently reliable is essential for drawing meaningful conclusions that can advance medical knowledge and practice.
This article has explored the critical roles of accuracy and precision, highlighting their impact on data quality, regulatory compliance, and patient safety. The discussion illustrates that inaccuracies can lead to harmful consequences, including misleading conclusions regarding treatment efficacy and regulatory failures. Moreover, the implementation of effective strategies—such as robust training, advanced technologies, and a culture of open communication—has been identified as vital for enhancing these qualities in clinical trials.
Ultimately, prioritizing accuracy and precision transcends mere procedural requirements; it is a moral imperative that safeguards patients and fosters trust in medical research. A commitment to uphold these standards will not only improve the quality of clinical outcomes but also contribute to the overall advancement of healthcare. Therefore, it is crucial for all stakeholders in clinical research to embrace these principles and actively seek ways to enhance them, ensuring that the future of medical research is built on a solid foundation of reliable and trustworthy data.
What is the definition of accuracy in clinical research?
In clinical research, accuracy is defined as the degree to which a measured value aligns with a known standard. It indicates that recorded measurements closely match the true values of the subjects being studied.
How is precision defined in the context of clinical research?
Precision refers to the consistency or reliability of repeated measurements. It suggests that repeated measurements yield similar results, regardless of whether those results are close to the true value.
Why is it important to understand the distinction between accuracy and precision?
Understanding the distinction is crucial for evaluating the reliability of medical data and outcomes. Both accuracy and precision are essential criteria for validating research findings and ensuring their applicability to real-world scenarios, which ultimately influences patient safety and treatment efficacy.
What are the possible combinations of accuracy and precision in measurements?
Measurements can be categorized as: both accurate and precise, accurate but not precise, precise but not accurate, or neither.
What services does Bioaccess® provide to uphold accuracy and precision in clinical research?
Bioaccess® offers comprehensive trial management services, including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting.
What are the consequences of inaccuracies in clinical research?
Inaccuracies can lead to flawed insights, regulatory setbacks, and potentially harm to patients, making it imperative that both accuracy and precision are prioritized in clinical research.
What do statistics reveal about error rates in clinical data entry?
Statistics indicate that error rates for single-information entry can range from 0.29% to 2.784 mistakes per 10,000 fields, highlighting the need for rigorous management practices in clinical research.
How does the case study titled 'The Importance of Accuracy and Precision Define in Clinical Trials' contribute to understanding these concepts?
The case study illustrates how accuracy and precision are essential for ensuring information integrity and supporting regulatory compliance in clinical trials.