


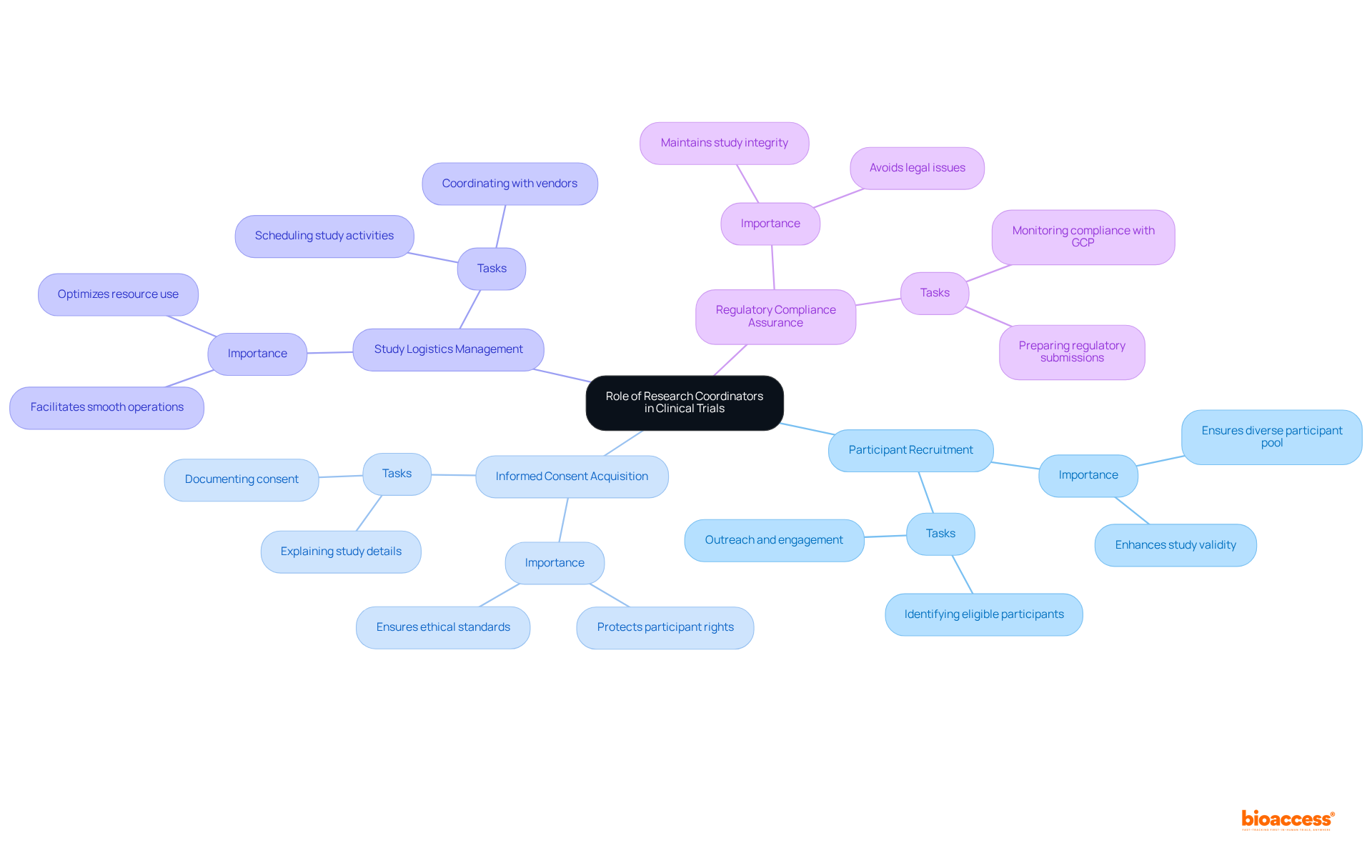
Research coordinators play a pivotal role in the success of clinical trials, serving as the primary liaison among the research team, participants, and regulatory authorities. They manage essential tasks, including:
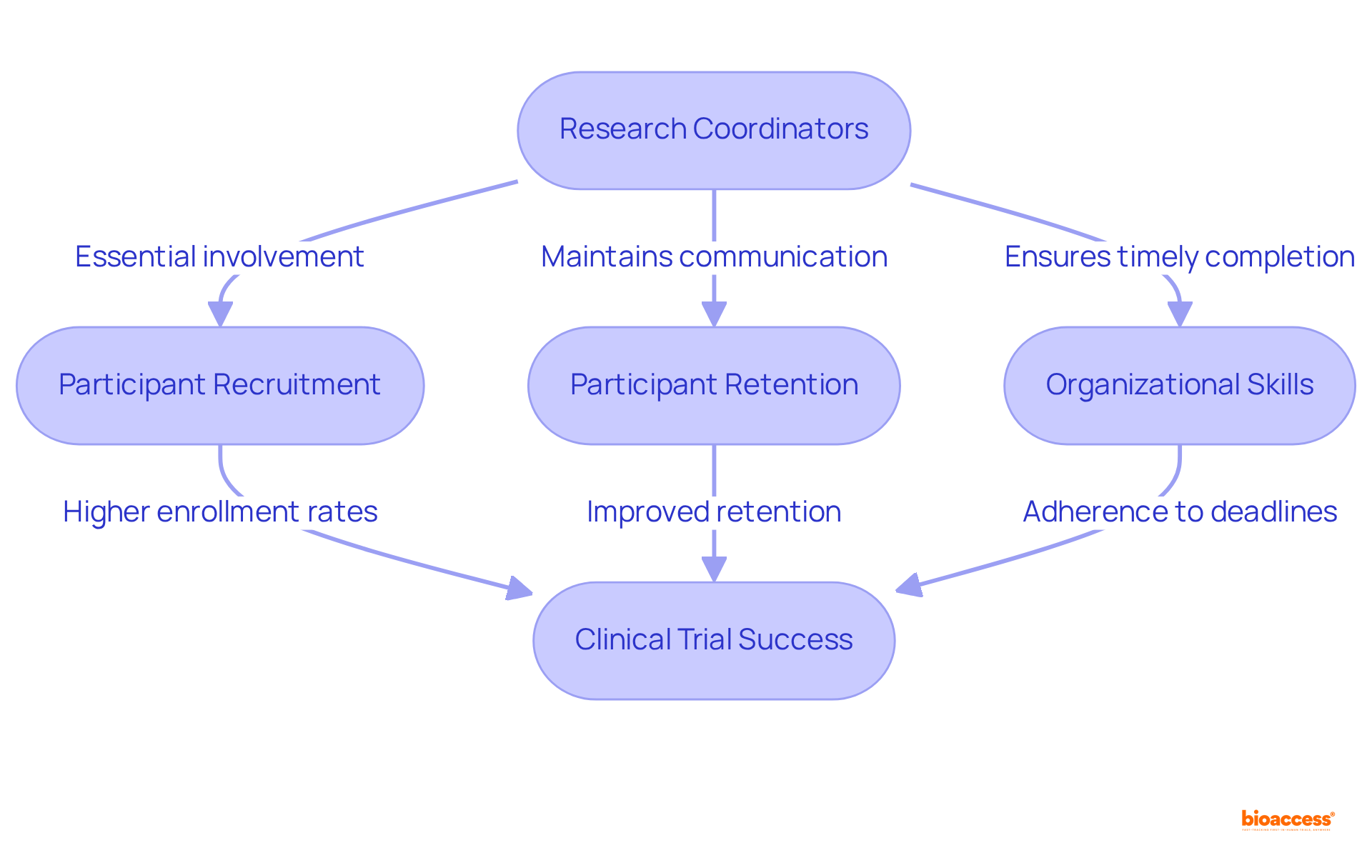
Their exceptional organizational skills and ability to foster collaboration significantly enhance participant retention and effectively address challenges. This, in turn, contributes to timely study completion and adherence to regulatory standards. Ultimately, these factors lead to more reliable trial outcomes.
Research coordinators serve as the backbone of clinical trials, orchestrating the intricate dance between researchers, participants, and regulatory bodies. Their multifaceted roles encompass everything from participant recruitment to ensuring compliance with stringent regulations, making them indispensable for the success of any medical study. Yet, despite their critical contributions, these professionals face a myriad of challenges that can hinder their effectiveness.
How can the industry better support research coordinators to enhance clinical trial outcomes and address the pressing issues they encounter?
Research coordinators play a crucial role in the successful implementation of clinical studies, serving as the primary liaison among the research team, participants, and regulatory authorities. Their responsibilities encompass a broad array of tasks, including:
By meticulously documenting all procedures and outcomes, research coordinators uphold the integrity of the study, ensuring adherence to Good Clinical Practice (GCP) guidelines. Moreover, research coordinators foster collaboration among team members, which facilitates process optimization and addresses challenges that may arise during the study.
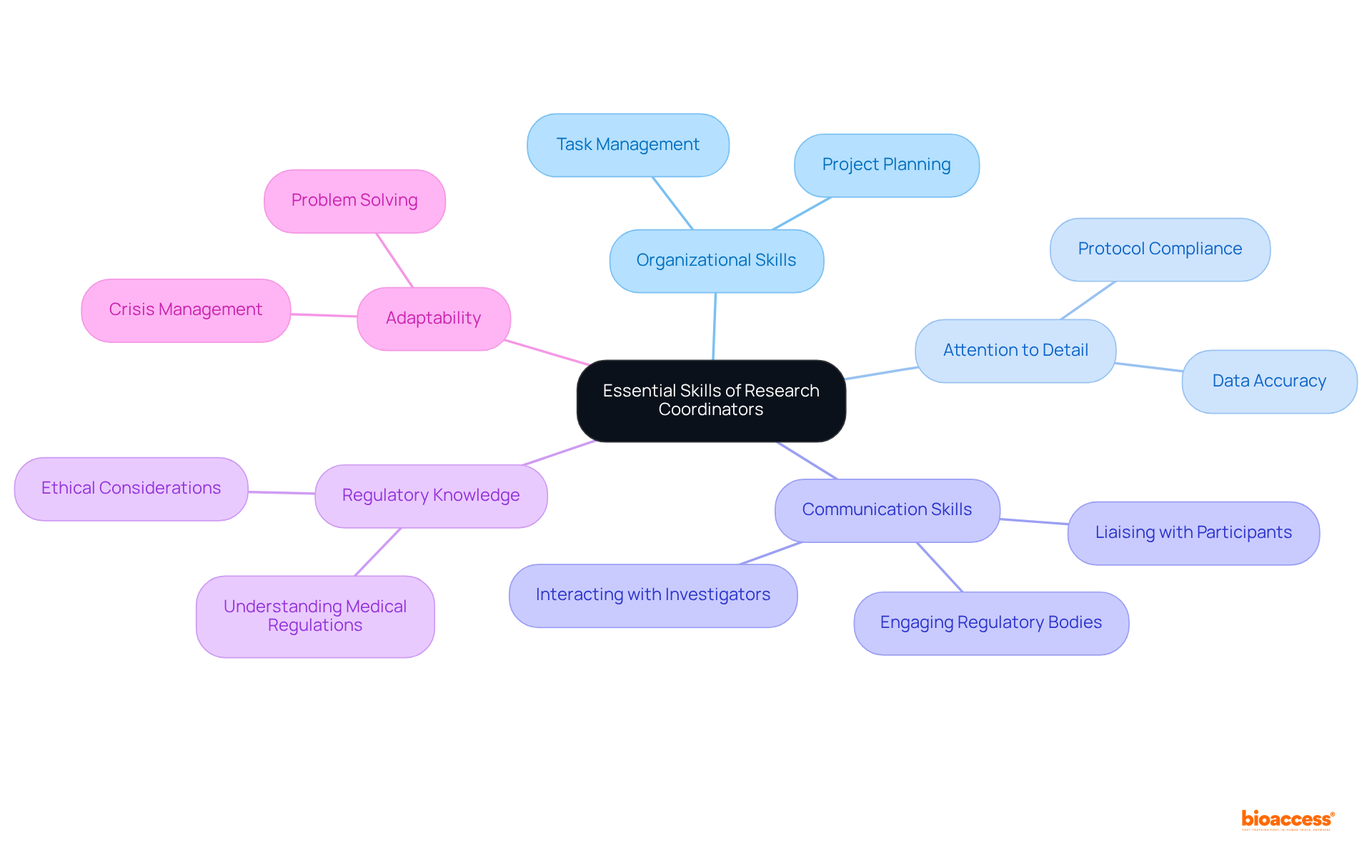
Research managers must possess a diverse skill set to navigate the complexities of medical trials effectively. Essential competencies include:
Effective communication skills are crucial for liaising with participants, investigators, and regulatory bodies. Additionally, a comprehensive understanding of medical study regulations and ethical considerations is vital. Research managers often benefit from certifications such as the Certified Clinical Research Manager (CCRM), which validate their expertise and commitment to maintaining high standards in medical studies. Their ability to adapt to changing circumstances and resolve issues efficiently is also critical for ensuring success in these trials.
The efficiency of study managers is pivotal to the success of clinical studies. Their involvement in participant recruitment is essential, as they frequently act as the first point of contact for potential participants, alleviating concerns and answering questions. Furthermore, research managers are instrumental in ensuring participant retention by maintaining regular communication and providing support throughout the study process. Their organizational skills are vital for the timely completion of study milestones, which is crucial for adhering to regulatory deadlines and securing funding.
Studies have shown that projects led by committed and proficient managers experience higher enrollment rates and fewer protocol deviations, ultimately leading to more reliable and valid outcomes.
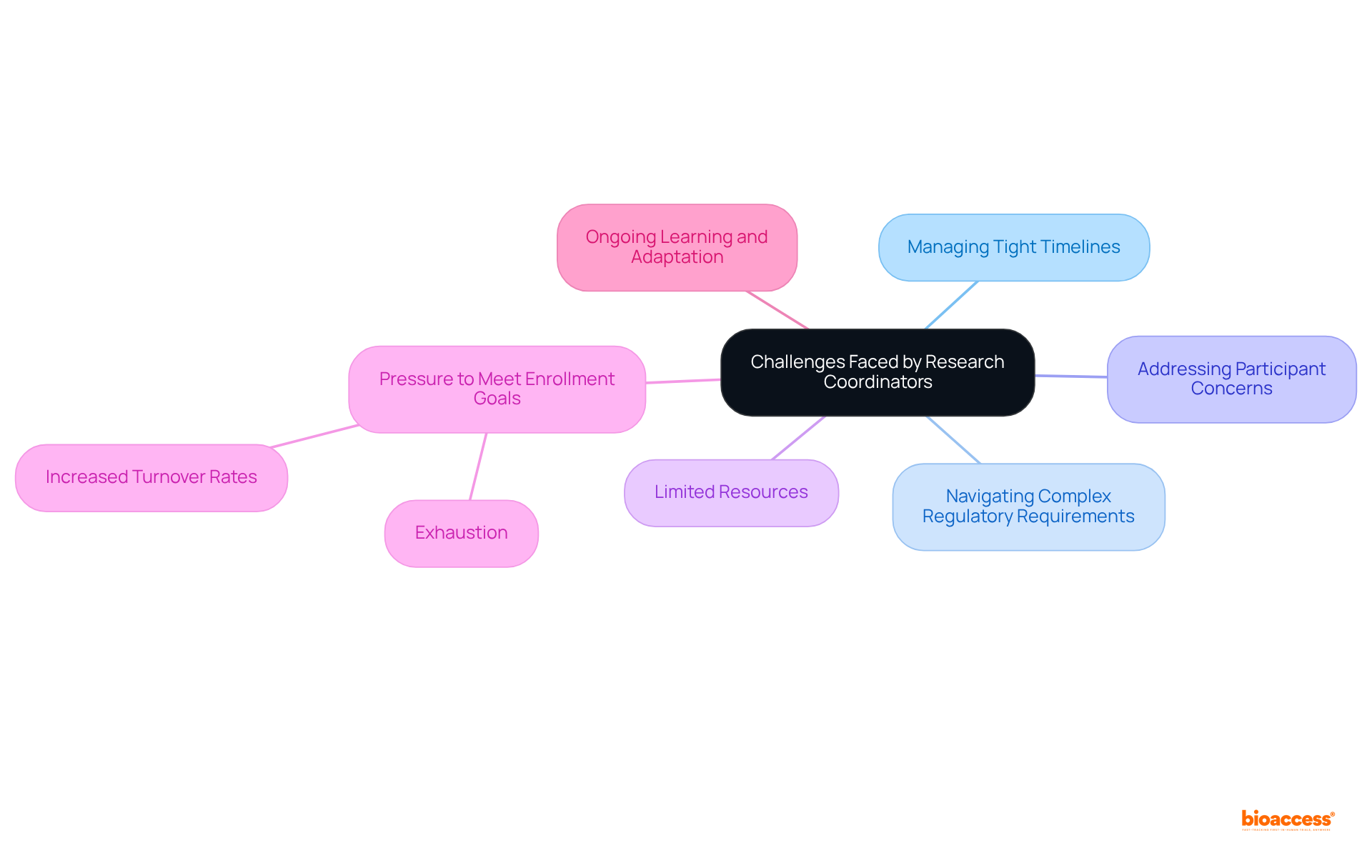
Despite their essential role, study managers encounter numerous challenges within the medical investigation environment. These challenges include:
Furthermore, they often operate with limited resources, which strains their capacity to effectively oversee multiple studies simultaneously. The pressure to meet enrollment goals can lead to exhaustion and increased turnover rates among study managers, complicating project oversight further. Additionally, the evolving nature of medical studies, characterized by the integration of new technologies and methodologies, necessitates ongoing learning and adaptation. Addressing these challenges is crucial for ensuring that research coordinators can effectively fulfill their roles and contribute to the success of clinical trials.
Research coordinators are indispensable to the success of clinical trials, serving as the vital link between research teams, participants, and regulatory agencies. Their multifaceted role encompasses participant recruitment, informed consent, study logistics, and compliance with regulations. By ensuring meticulous documentation and fostering collaboration, they uphold the integrity of clinical studies and facilitate the achievement of research objectives.
The essential skills and competencies that research coordinators must possess include strong organizational abilities, keen attention to detail, and effective communication. These skills are critical in managing the complexities of clinical trials and addressing challenges such as tight timelines and participant concerns. Furthermore, the impact of research coordinators on trial success is underscored, with evidence showing that their involvement leads to higher enrollment rates and fewer protocol deviations.
Ultimately, the role of research coordinators extends beyond administrative tasks; they are central to the advancement of medical research. As the landscape of clinical trials evolves, it is crucial to recognize and support the contributions of research coordinators. Investing in their training and addressing the challenges they face will not only enhance their effectiveness but also improve the overall success of clinical trials, paving the way for groundbreaking medical advancements.
What is the primary role of research coordinators in clinical trials?
Research coordinators serve as the primary liaison among the research team, participants, and regulatory authorities, playing a crucial role in the successful implementation of clinical studies.
What are some of the key responsibilities of research coordinators?
Key responsibilities include participant recruitment, informed consent acquisition, study logistics management, and regulatory compliance assurance.
How do research coordinators ensure the integrity of a clinical study?
They uphold the integrity of the study by meticulously documenting all procedures and outcomes, ensuring adherence to Good Clinical Practice (GCP) guidelines.
In what way do research coordinators contribute to team collaboration?
Research coordinators foster collaboration among team members, which facilitates process optimization and addresses challenges that may arise during the study.