


This article has a clear purpose: to provide direct and transparent answers to the most frequently asked questions about the International Accrediting Organization for Clinical Research (IAOCR) and Global Clinical Site Assessment (GCSA) certification. We will address the certification process, its tangible value, alignment with global standards, and the investment structure.
IAOCR/GCSA certification represents the gold standard in clinical research. It is an accreditation system adopted by leading organizations in more than 40 countries and is supported by regulators like the UK's MHRA, with active discussions underway with the United States FDA.
Currently, the Americas are experiencing a historic moment in clinical research certification. This transformation is being led by pioneering sites that are setting a new standard of excellence in the region:
The mention of these sites is not a simple client list; it represents a coordinated regional movement. It demonstrates a collective shift toward international quality standards across Latin America and the Caribbean, creating an opportunity for new sites to join this vanguard group and not only improve their own operations but also become part of a prestigious network of leaders.
One of the most common questions is about the nature of the process. It is essential to understand that IAOCR/GCSA certification is not a traditional, punitive audit. It is a collaborative, supportive, and evidence-based journey designed to build capabilities and foster a lasting culture of quality, not just to find faults.
The GCSA certification process for a research site is comprehensive and typically takes 30 to 120 days, depending on the site's size, complexity, and level of preparedness. The methodology focuses on a holistic review covering seven critical operational areas identified by sponsors and CROs as fundamental to executing high-quality trials:
The following chart breaks down the process to eliminate any uncertainty and demonstrate its collaborative nature.
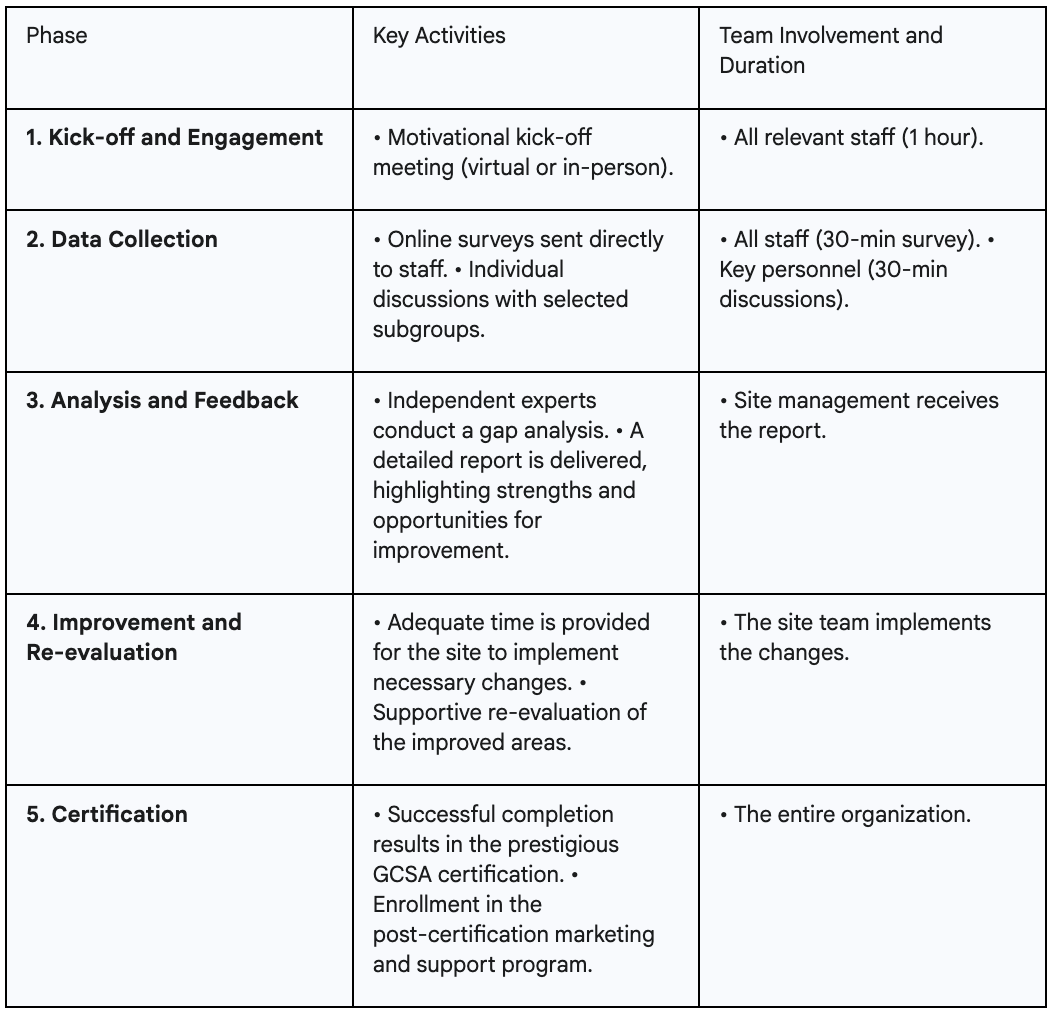
Sustainable quality is the result of both robust systems and competent, engaged people. Therefore, the IAOCR framework also offers accreditation for individual professionals, recognizing that operational excellence depends on the verified expertise of the team.
The verification system is unique and robust, consisting of two stages:
There are accreditation levels that map out a clear professional development pathway:
This dual approach, addressing both site processes (GCSA) and staff competence (IAOCR), is a strategic decision. It acknowledges that the best standard operating procedures (SOPs) fail if staff are not engaged or competent. Likewise, a great team can be limited by poor systems. The IAOCR/GCSA framework addresses both sides of the quality equation, fostering a cultural transformation that goes far beyond a simple certification.
The value of IAOCR/GCSA certification is not theoretical. It is backed by documented case studies that demonstrate a tangible return on investment in operational efficiency, financial performance, and market reputation.
Certified sites report substantial and measurable improvements that directly impact their performance and sustainability.
The voices of certified sites are the most powerful testament to this value:
Individual accreditation offers clear benefits for professional career development:
As Dr. Pedro Martinez-Clark explains: "I pursued IAOCR Investigator Accreditation to gain independent recognition of my skills and commitment to globally accepted best practice standards. This accreditation benefits me as a clinical research professional and positively reflects on my organization, team, and stakeholders."
Ultimately, all these benefits converge on a final goal: becoming a preferred partner for sponsors and CROs. Certification creates a virtuous cycle: the process improves operations; better operations produce higher-quality data with fewer errors; this reduces costs and risks for sponsors, increasing their confidence; and greater confidence leads to the award of more and better clinical trials, which in turn increases revenue and profitability.
This cycle is not left to chance. IAOCR actively accelerates it through robust post-certification marketing support. You don't just receive a credential; you are given a megaphone to announce that achievement to the global industry. This transforms certification from a passive qualification into an active business development tool.
IAOCR/GCSA certification is built on the foundation of the most critical international standards. This ensures that certified sites and professionals operate at a universally accepted level of quality, affirmatively answering the question about alignment with Good Clinical Practice (GCP) and ISO 9001:2015.
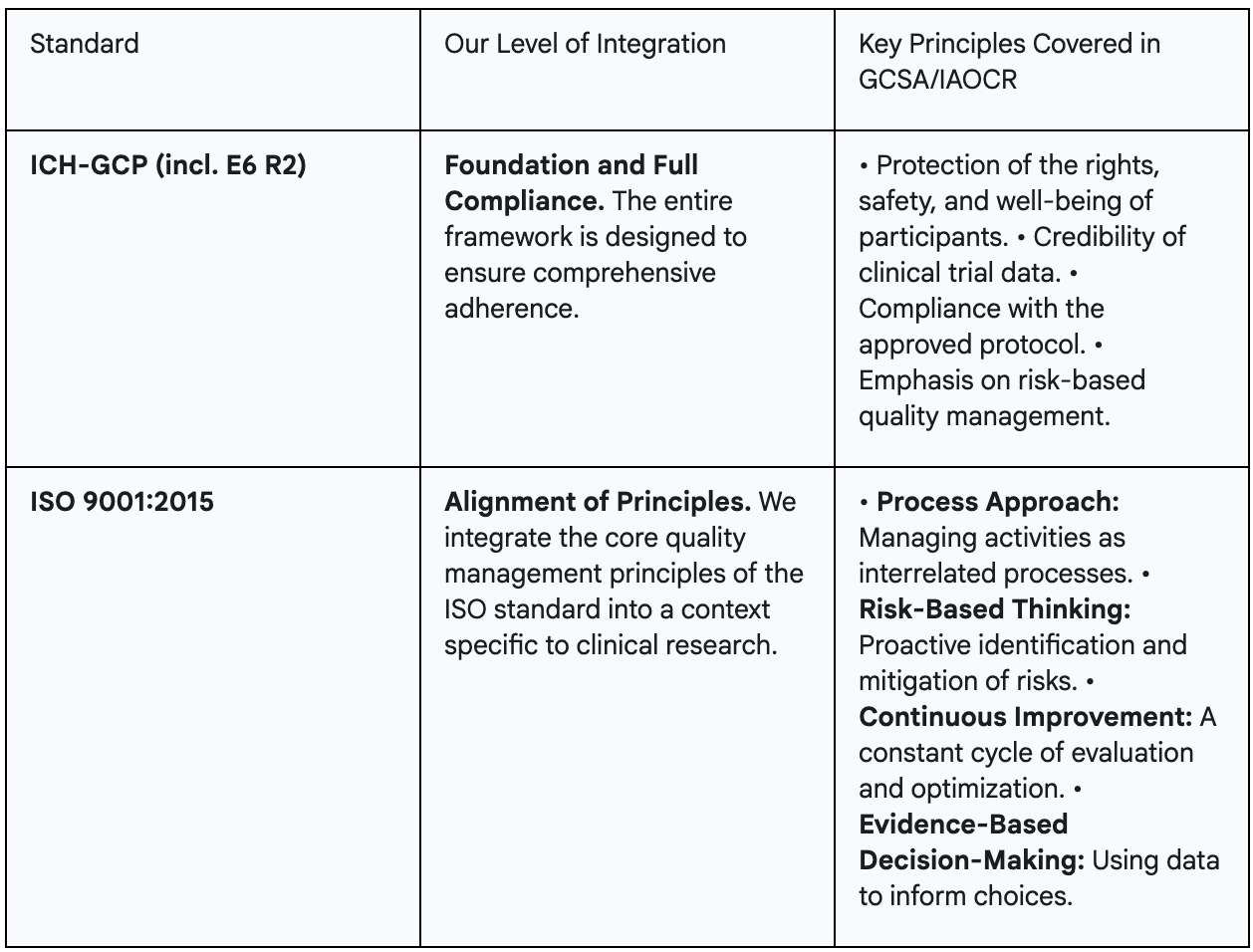
Compliance with the International Council for Harmonisation's Good Clinical Practice (ICH-GCP) guidelines is the non-negotiable core of the certification. This includes adherence to the latest addenda, such as ICH E6(R2), which is essential for demonstrating modern, risk-based approaches to quality management—a key requirement for global sponsors.
GCSA is not an ISO 9001 certification. Instead, a strategic choice has been made: to distill the most powerful concepts from the ISO standard and apply them practically to the clinical research environment. This allows sites to benefit from world-class systems thinking without the bureaucratic burden that a generic quality management system can entail.
The alignment goes beyond these two standards. The framework is designed to meet the requirements of major international regulatory agencies, ensuring that a certified site is prepared for inspections from:
This multi-layered alignment creates a strategic asset of immense value: a "universal passport" for quality. A GCSA-certified site can present a single, credible credential that satisfies the quality requirements of a wide range of sponsors and regulators from different regions. This drastically reduces the "audit burden"—the time and resources spent preparing for and hosting multiple, often redundant, audits from different partners—simplifying international business development and positioning the site as an easy and reliable partner to work with.
We address the question of cost directly and transparently. It is not a "cost," but an "investment in excellence." The structure is designed to be fair, scalable, and clear, with a price directly related to the scope of work required for each organization.
There is no single price because the investment is tailored to the specific characteristics of each organization. The factors that influence the investment include:
The pricing model for site certification is based on team size, ensuring its scalability:
To shift the conversation from "cost" to "return on investment," it is crucial to understand the comprehensive value included in the price. Your investment includes:
For professionals, there are flexible options that offer accessible entry points to build a foundation of quality, from fundamental programs (like ICH-GCP) to role-based professional accreditations (Coordinator, Investigator, etc.).
By detailing everything included in the investment—especially the marketing support that connects directly to revenue-generating benefits—the business case for certification becomes self-evident. The question is no longer "how much does it cost?" but "what is the return on this strategic investment?".
In summary, we have provided clear answers to your questions: the process is collaborative, the value is demonstrable, the alignment is global, and the investment is transparent and scalable.
Joining the ranks of certified sites like the Hospital Internacional de Colombia, amavita Heart and Vascular Health®, Hidra Investigaciones Clínicas, and C&M Research is an opportunity to be at the forefront of the quality transformation in the Americas.
IAOCR/GCSA certification is more than a credential; it is a "passport to the future of world-class clinical research." It is a commitment to excellence that reduces operational risks, attracts top-tier partners, and ensures a sustainable competitive advantage. IAOCR Americas is not a vendor, but a strategic partner on this journey.
To begin your own transformation, the next steps are simple:
With dedicated support specifically for the Americas region, including materials and assessments fully in Spanish, the path to excellence in clinical research has never been more accessible or relevant.