


Navigating the intricate landscape of labeling and packaging regulations in Bosnia is essential for companies looking to successfully launch their products. With stringent compliance requirements and a rising consumer demand for transparency and sustainability, businesses must prioritize adherence to local laws while aligning their practices with market expectations. But here's the challenge: how can companies effectively balance regulatory compliance with innovative packaging solutions that resonate with consumers? This article delves into ten essential rules that not only ensure compliance but also enhance product appeal in the competitive Bosnian market.
bioaccess harnesses its extensive expertise to navigate the regulatory landscape of the region, securing swift ethical approvals for clinical trials - typically within just 4-6 weeks. This expedited process is vital for innovators in Medtech, Biopharma, and Radiopharma, enabling them to initiate their studies without unnecessary delays.
In a competitive environment where timely access to clinical data can significantly influence development and market entry strategies, bioaccess's commitment to rapid approvals enhances the likelihood of favorable clinical trial outcomes. The ability to launch studies quickly not only accelerates innovation but also positions clients advantageously within the evolving healthcare landscape of the region, where the demand for effective therapies continues to rise.
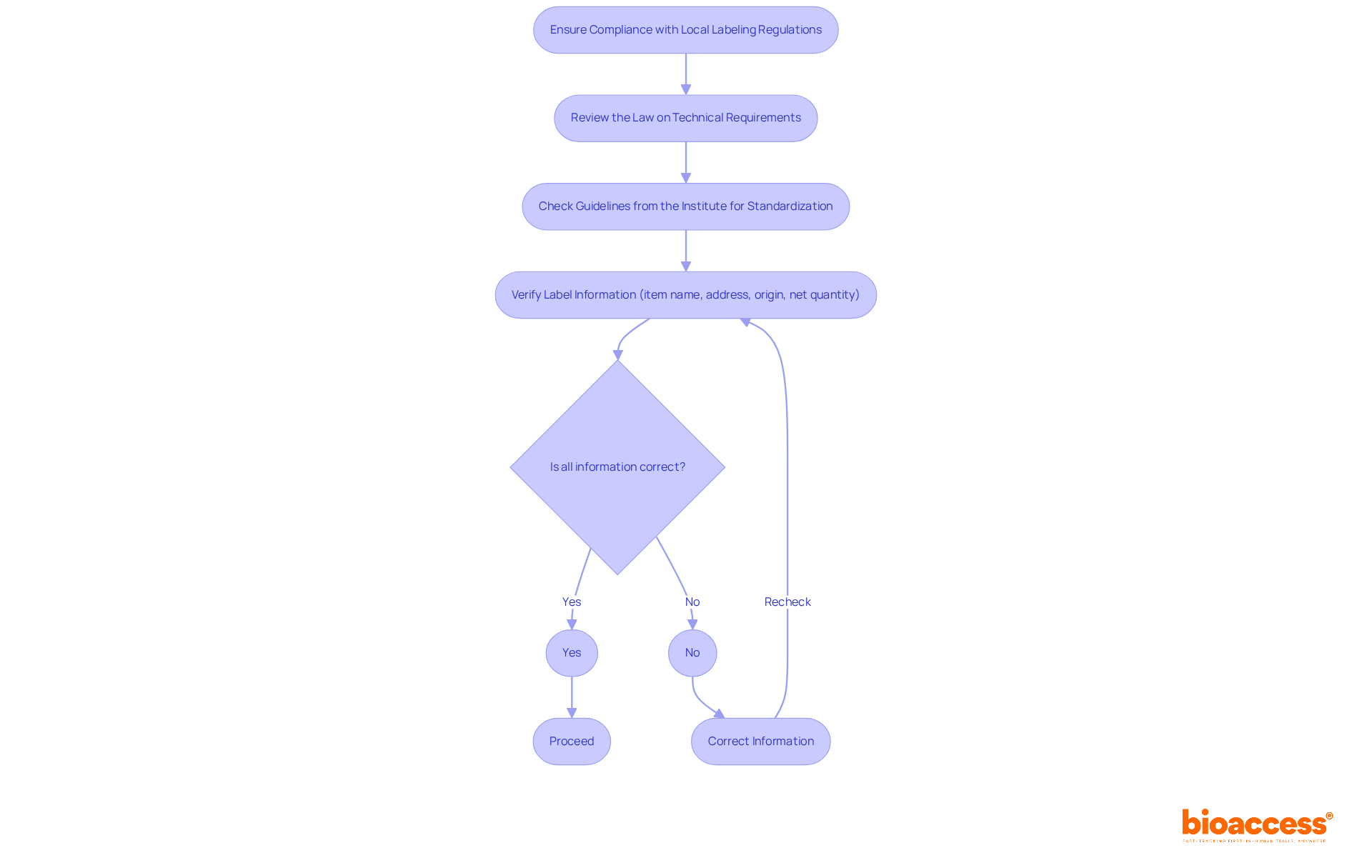
In Bosnia, compliance with the labeling and packaging rules for ips in Bosnia is not just important; it’s essential for successful market entry. Labels must include critical information such as the item name, the importer’s address, country of origin, and net quantity. Compliance with these requirements is vital; failure to do so can result in serious consequences, including rejection at customs and potential legal penalties. Companies should thoroughly review the Law on Technical Requirements and the guidelines issued by the Institute for Standardization of Bosnia and Herzegovina to ensure they meet all necessary standards.
Frequent causes for item rejection at customs often stem from inadequate marking practices. For example, missing or incorrect information can lead to delays and additional costs. There have been instances where companies faced penalties due to non-compliance with marking regulations, underscoring the need for vigilance in this area. Furthermore, companies must be aware of customs challenges, such as missing declarations of origin for shipments valued under EUR 6,000, which can complicate the clearance process. By prioritizing adherence to the labeling and packaging rules for ips in Bosnia, companies can navigate the regulatory landscape more effectively and avoid costly setbacks.
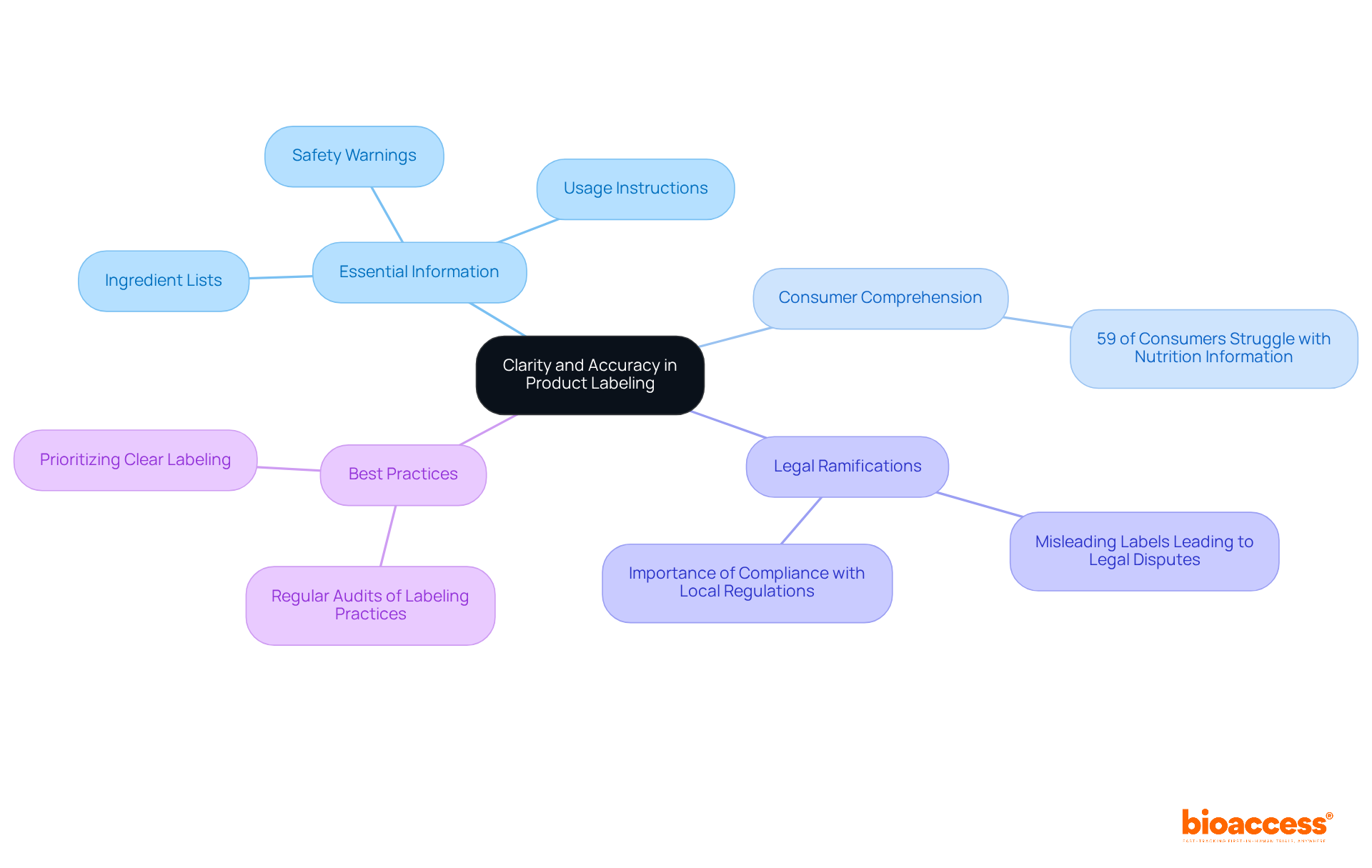
Labels must be meticulously crafted to communicate essential information with clarity and precision. This includes ingredient lists, usage instructions, and safety warnings. Misleading or ambiguous labels not only confuse consumers but can also lead to significant legal ramifications. Did you know that 59% of consumers struggle to comprehend nutrition information? This statistic underscores the necessity for clear displays. Consumer safety proponents emphasize that precise labeling is essential for building trust; when buyers can easily understand what they are acquiring, their confidence in the item rises.
Misleading labels have resulted in legal disputes in Bosnia, underscoring the importance of adhering to the labeling and packaging rules for ips in Bosnia. For instance, products labeled as 'natural' without proper definitions have faced scrutiny and legal action. To mitigate risks, companies should conduct regular audits of their labeling practices, ensuring compliance with local regulations and that all information remains current. By prioritizing clear labeling, businesses can enhance consumer trust and avoid potential legal disputes.
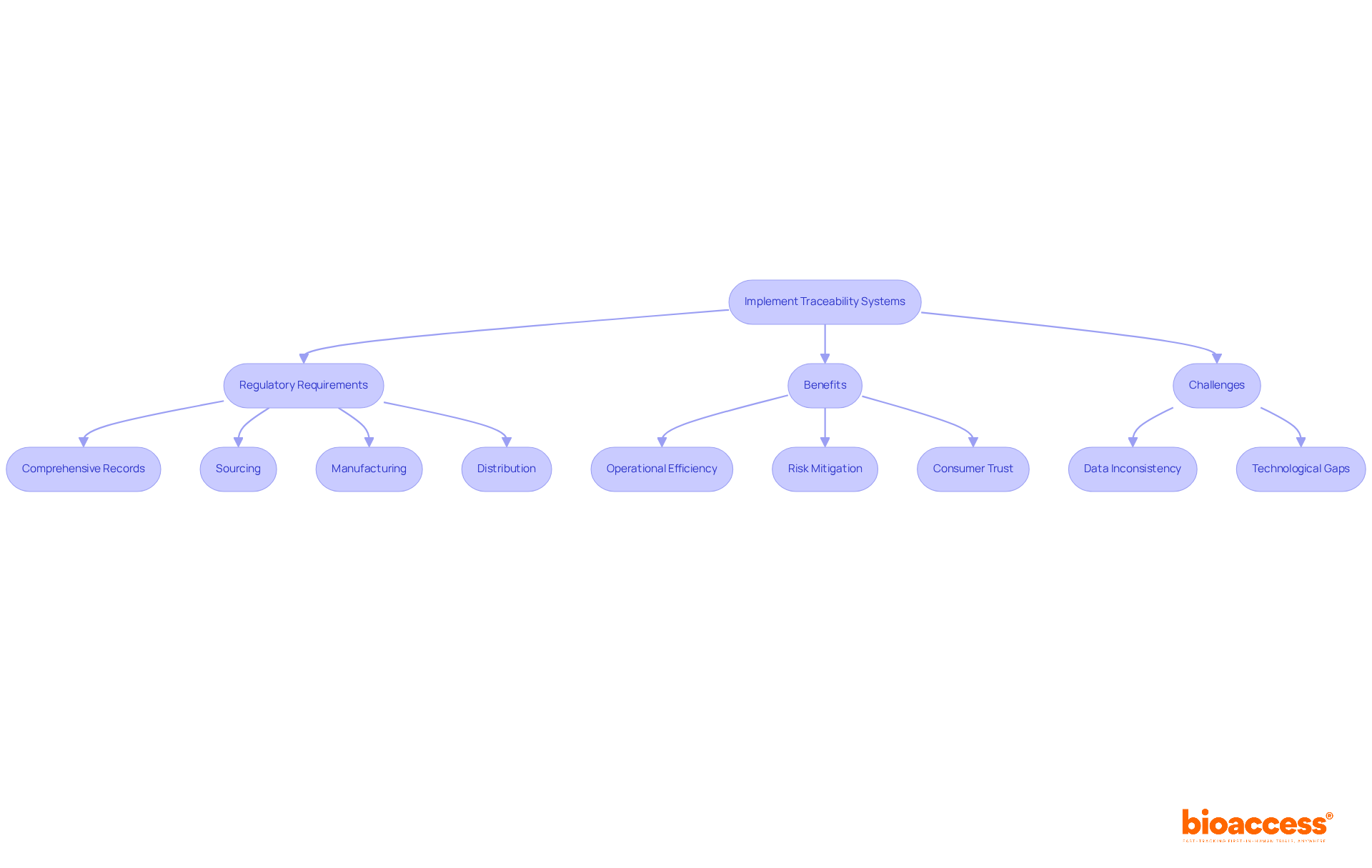
Traceability systems are essential for companies operating in Bosnia, enabling effective tracking of items throughout the supply chain. This capability not only allows for swift identification and resolution of issues but also significantly enhances safety. Starting in 2025, regulatory requirements will mandate that businesses maintain comprehensive records detailing sourcing, manufacturing processes, and distribution channels. Such transparency is not just a best practice; it is vital for compliance with local laws and for building consumer trust.
Experts in the supply chain, including Chad Clayborne, assert that effective traceability boosts operational efficiency and mitigates risks associated with product recalls. Accurate data ensures immediate responses to safety issues during recalls, illustrating that companies adhering to traceability regulations not only fulfill legal obligations but also enhance their brand reputation and consumer confidence.
However, challenges such as data inconsistency and technological gaps can impede the implementation of these systems. It is crucial for companies to proactively address these issues to fully leverage the benefits of traceability.
As consumer awareness of environmental issues grows, the shift to sustainable packaging materials has become crucial for companies in the region. Businesses are now expected to adopt biodegradable, recyclable, or compostable materials to meet consumer preferences and adhere to evolving regulations. Embracing sustainable packaging not only boosts brand reputation but also ensures compliance with increasingly stringent EU directives on packaging waste.
Statistics reveal that a significant number of consumers in the region actively seek eco-friendly packaging options, mirroring a global trend where 68% of customers have recently chosen products based on sustainability credentials. Local companies are responding to this demand; for example, several Bosnian brands have started integrating biodegradable packaging solutions, demonstrating their commitment to environmental responsibility.
As environmental advocates stress, sustainable packaging is not merely a trend but a vital evolution in business practices. This underscores the importance of innovation in reducing ecological footprints and fostering a healthier planet.

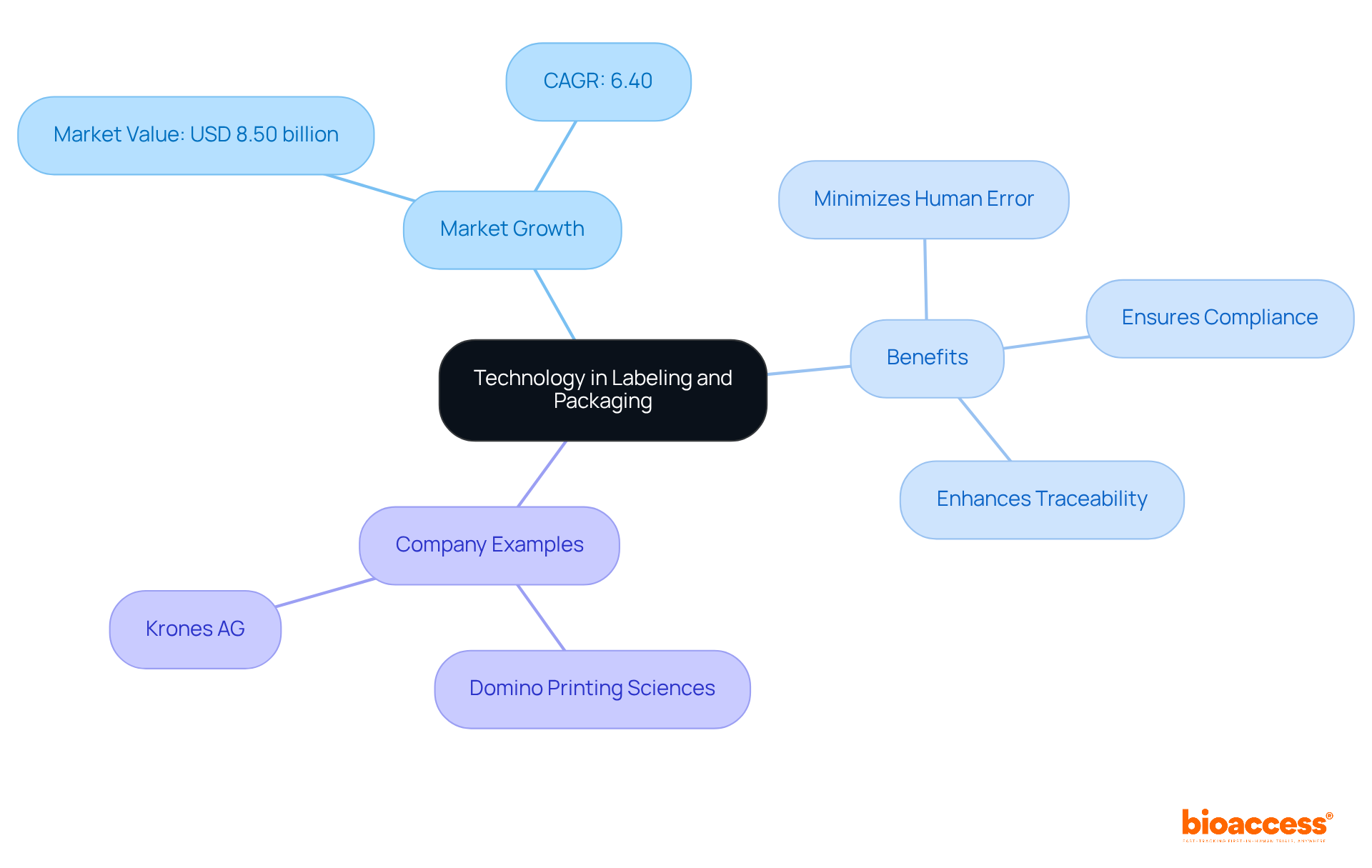
Implementing automated tagging systems and advanced digital printing technologies can significantly improve the labeling and packaging rules for ips in Bosnia. The global automatic tagging machine market, valued at USD 8.50 billion in 2024, is projected to grow at a CAGR of 6.40% from 2025 to 2032. Such innovations not only minimize human error but also ensure compliance with regulatory standards and allow for quick adjustments to the labeling and packaging rules for ips in Bosnia.
For example, companies like Domino Printing Sciences highlight that these systems streamline operations and provide a substantial competitive edge in the market. By investing in these solutions, Bosnian companies can optimize workflows, enhance traceability, and adhere to the labeling and packaging rules for ips in Bosnia to meet the increasing consumer demand for transparency and quality.
As Krones AG notes, "The Wrap-Around Labelling Machines segment held the largest market revenue share of 38.2% in 2024, driven by its versatility in applying seamless, high-quality labels across diverse industries such as food and beverage, pharmaceuticals, and cosmetics.
Effective collaboration among all stakeholders - including manufacturers, regulatory bodies, and distributors - is crucial for successful product launches in Bosnia. By nurturing strong relationships, companies can enhance adherence to the labeling and packaging rules for IPS in Bosnia, enabling smoother market entry. Consistent communication is essential; it guarantees that all parties are aligned on expectations and requirements, ultimately resulting in enhanced adherence rates.
Ana Criado, with her extensive experience in regulatory affairs, emphasizes that leveraging expert knowledge can significantly enhance adherence outcomes. For instance, organizations that prioritize open communication frequently observe an 18% increase in productivity, a factor that can greatly influence the effectiveness of launches. Industry leaders underscore that clear communication is the linchpin of teamwork, particularly in adhering to the labeling and packaging rules for IPS in Bosnia, highlighting its importance in navigating the complexities of regulatory compliance.
Successful collaborations in the Bosnian Medtech market demonstrate that when stakeholders cooperate effectively, they can achieve remarkable results. This paves the way for innovative solutions to reach the market more swiftly, showcasing the undeniable impact of strategic partnerships in clinical research.

Understanding consumer preferences is crucial for developing effective packaging strategies. Companies must conduct market research to pinpoint what information resonates most with consumers on labels - be it ingredient transparency or sustainability claims. This research not only guides branding choices but also ensures that offerings resonate with the intended audience in the region. By prioritizing consumer insights, businesses can create packaging that truly connects, fostering trust and loyalty.

Regular training sessions for staff on marking criteria and regulations are crucial for maintaining compliance and ensuring the quality of goods. Companies in Bosnia must implement comprehensive training programs that encompass:
By leveraging the expertise of professionals like Ana Criado, who possesses extensive experience in regulatory affairs and biomedical engineering, these training initiatives can become significantly more effective. This investment in staff education not only helps prevent costly errors but also enhances overall product integrity, making it a vital step for any organization committed to excellence.
Regular audits of marking processes are crucial for pinpointing areas needing improvement and ensuring compliance with regulations. By establishing a routine audit schedule, companies can effectively assess labeling accuracy, adherence to local laws, and overall effectiveness. These audits not only help organizations adapt to evolving regulations and consumer expectations but also significantly enhance product outcomes. As quality assurance experts from Bermingham, Castleman & Pierce Inc. assert, 'Quality is never an accident; it is always the result of high intention, sincere effort, intelligent direction, and skillful execution.' This underscores the necessity of regular audits in maintaining high standards of compliance.
Moreover, organizations can leverage proven methodologies like Lean and Six Sigma to streamline their auditing processes, fostering a culture where quality is a shared responsibility. For example, companies in Bosnia have successfully enhanced their compliance with the labeling and packaging rules for ips in bosnia through systematic audits, showcasing the tangible benefits of this approach. This not only highlights the effectiveness of regular audits but also encourages other organizations to consider similar strategies in their operations.

Navigating the labeling and packaging regulations for IPS in Bosnia is essential for businesses striving to thrive in this competitive market. Adhering to local compliance standards not only facilitates smooth market entry but also builds consumer trust and enhances product safety. By grasping and implementing the crucial guidelines outlined in this article, companies can mitigate risks associated with non-compliance and position themselves advantageously within the industry.
Key insights highlight the significance of:
Each of these elements is vital in ensuring compliance with local regulations while meeting consumer expectations. Moreover, leveraging technology and fostering collaboration among stakeholders can significantly streamline processes and improve overall outcomes.
Ultimately, embracing these best practices transcends mere compliance; it drives innovation and sustainability in the Bosnian market. Companies are urged to take proactive steps in refining their labeling and packaging strategies to align with evolving regulations and consumer preferences. By doing so, they can enhance their brand reputation, ensure product safety, and contribute to a more responsible and transparent marketplace.
What is bioaccess and how does it support clinical research in Bosnia?
Bioaccess is an organization that leverages its expertise to navigate the regulatory landscape in Bosnia, securing swift ethical approvals for clinical trials, typically within 4-6 weeks. This expedited process is crucial for innovators in Medtech, Biopharma, and Radiopharma, allowing them to commence their studies without unnecessary delays.
Why are fast approvals important for clinical trials?
Fast approvals are vital in a competitive environment where timely access to clinical data can significantly impact development and market entry strategies. Bioaccess's commitment to rapid approvals enhances the likelihood of favorable clinical trial outcomes and positions clients advantageously within the evolving healthcare landscape.
What are the key labeling requirements for products in Bosnia?
In Bosnia, labels must include essential information such as the item name, the importer’s address, country of origin, and net quantity. Compliance with these labeling and packaging rules is crucial for successful market entry.
What are the consequences of failing to comply with labeling regulations in Bosnia?
Failure to comply with labeling regulations can result in serious consequences, including rejection at customs and potential legal penalties. Inadequate marking practices are frequent causes of item rejection at customs, leading to delays and additional costs.
What challenges do companies face regarding customs in Bosnia?
Companies may encounter customs challenges, such as missing declarations of origin for shipments valued under EUR 6,000, which can complicate the clearance process. It is essential for companies to ensure compliance with all necessary standards to avoid these complications.
Why is clarity and accuracy important in product labeling?
Clarity and accuracy in product labeling are crucial for communicating essential information, such as ingredient lists, usage instructions, and safety warnings. Misleading or ambiguous labels can confuse consumers and lead to significant legal ramifications.
What are the risks associated with misleading labels in Bosnia?
Misleading labels can result in legal disputes, particularly for claims that lack proper definitions, such as products labeled as 'natural.' Companies must conduct regular audits of their labeling practices to ensure compliance and mitigate risks.
How can clear labeling enhance consumer trust?
Clear labeling helps consumers easily understand what they are purchasing, which builds their confidence in the product. Precise labeling is essential for fostering trust and avoiding potential legal disputes.