


The article titled "10 Key Insights on Paid Medical Trials for Participants" serves to provide essential information regarding the multifaceted nature of paid medical trials. It encompasses critical aspects such as:
Participants can anticipate a spectrum of compensation that varies according to trial phases, while rigorous safety protocols are in place to safeguard their well-being. Moreover, the significance of informed consent is underscored, ensuring that ethical standards are upheld throughout the research process.
In a world where medical advancements are crucial for improving healthcare, paid medical trials play a pivotal role in shaping the future of treatment options. These trials not only provide participants with the opportunity to access cutting-edge therapies but also offer financial compensation for their involvement.
However, navigating the complexities of eligibility, safety protocols, and compensation structures can be daunting for potential participants. What insights can be gained to empower individuals in making informed decisions about joining these trials, and how can they maximize their benefits while ensuring their safety?
This article delves into ten key insights that illuminate the landscape of paid medical trials, equipping readers with the knowledge to engage confidently in this vital aspect of medical research.
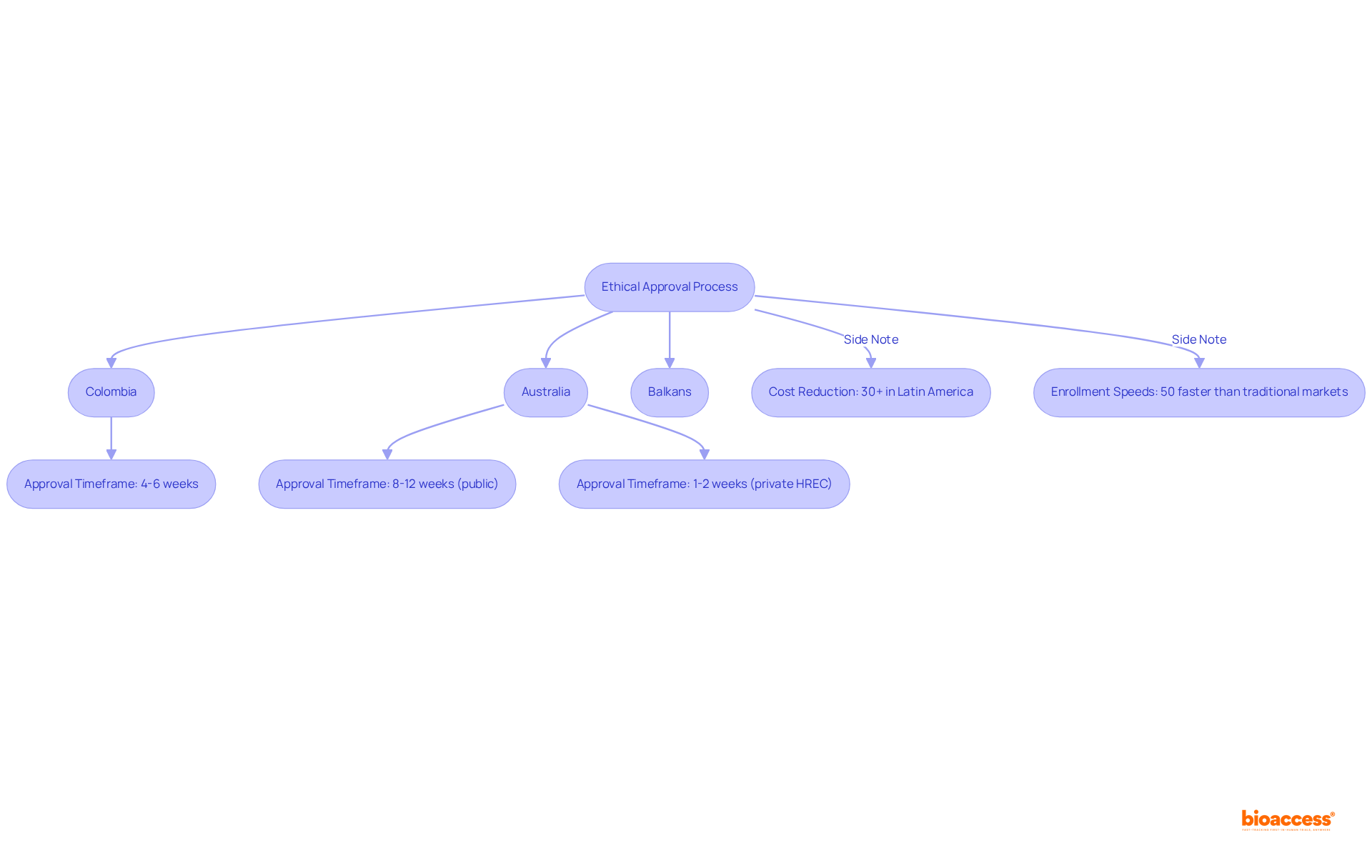
bioaccess® capitalizes on the regulatory efficiency found in Latin America, particularly Colombia, where cost reductions for studies can exceed 30% compared to North America and Western Europe. The diverse patient demographics in the Balkans, alongside Australia's streamlined ethical approval processes, significantly enhance our capacity to conduct clinical studies with exceptional speed. Participants can anticipate quicker enrollment and expedited access to innovative treatments, establishing bioaccess® as a frontrunner in the realm of paid medical trials.
In Colombia, ethical approvals are secured in just 4-6 weeks, allowing individuals to engage in studies that prioritize not only promptness but also adhere to rigorous standards of care and compliance. Looking ahead to 2025, the average duration for ethical approvals in Australia remains competitive; public hospitals typically require 8-12 weeks, while private Human Research Ethics Committees (HREC) can accelerate this to as little as 1-2 weeks.
This efficiency underscores the critical importance of timely ethical approvals in propelling health innovations, a sentiment echoed by industry leaders who recognize the essential role of swift regulatory pathways in enhancing patient access to new treatments. Remarkably, bioaccess® achieves enrollment rates that are 50% faster than those of traditional markets, further reinforcing its competitive advantage.
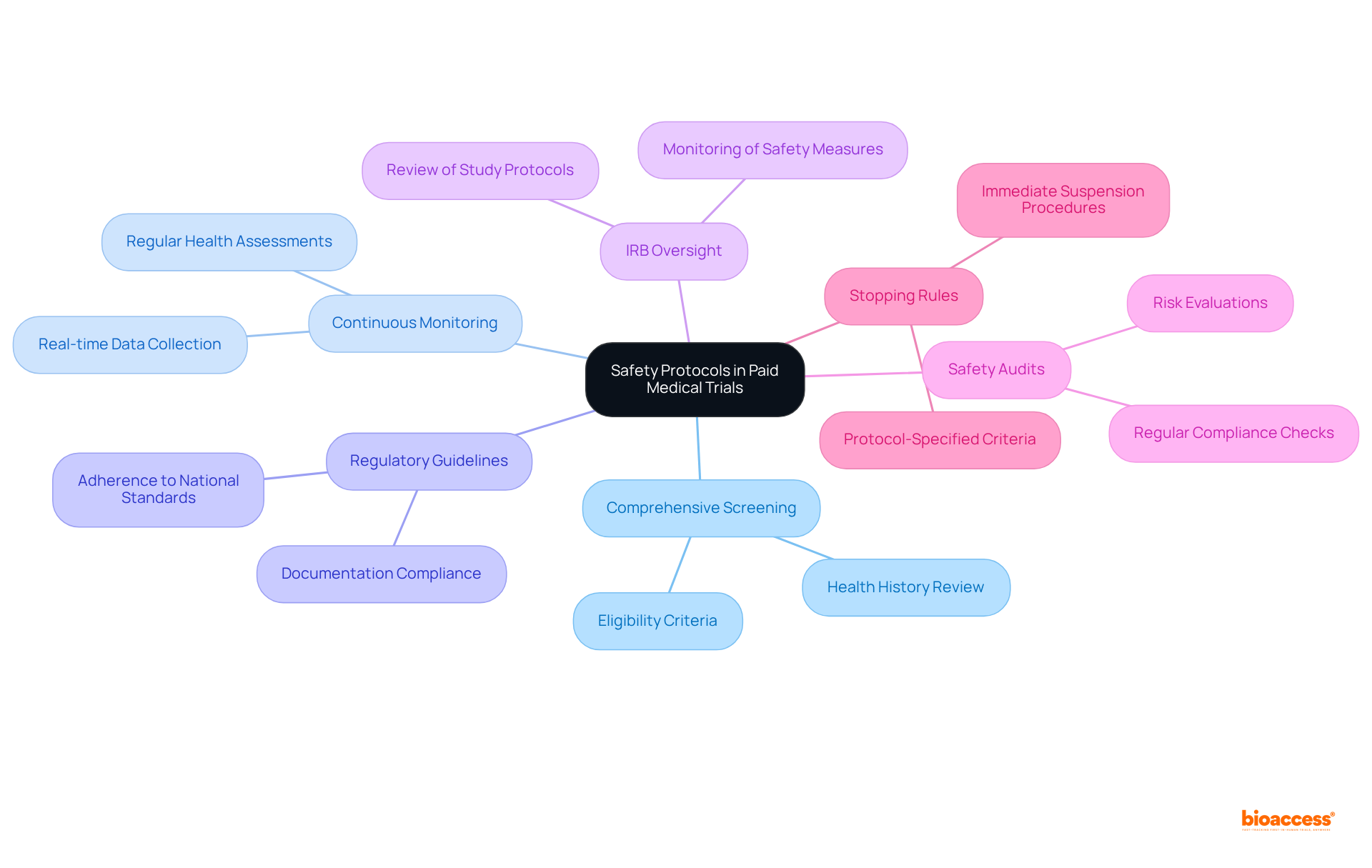
Safety measures in paid medical trials are meticulously designed to protect individuals throughout the research process. These measures encompass:
Institutional Review Boards (IRBs) oversee these studies, ensuring that all safety measures are implemented and that individuals are made aware of potential risks. In addition to these protocols, bioaccess underscores the importance of thorough study preparation and compliance assessments, verifying that all research documents align with national requirements prior to the study's initiation.
Regular safety audits and risk evaluations are conducted to mitigate unforeseen issues, prioritizing the well-being of participants. Current compliance rates with safety regulations in paid medical trials are impressively high, reflecting the industry's commitment to upholding rigorous safety standards.
Experts in clinical research assert that a proactive approach to subject safety not only protects individuals but also enhances the overall integrity of the research process. As one expert noted, 'Safety has to be everyone’s responsibility… everyone needs to know that they are empowered to speak up if there’s an issue.'
Furthermore, protocol-specified stopping rules are established to suspend the study in the event of an excessive number of safety incidents, reinforcing the commitment to participant protection. Through effective project management and vigilant monitoring, bioaccess guarantees that all facets of the study, including safety protocols, are executed with the utmost diligence.
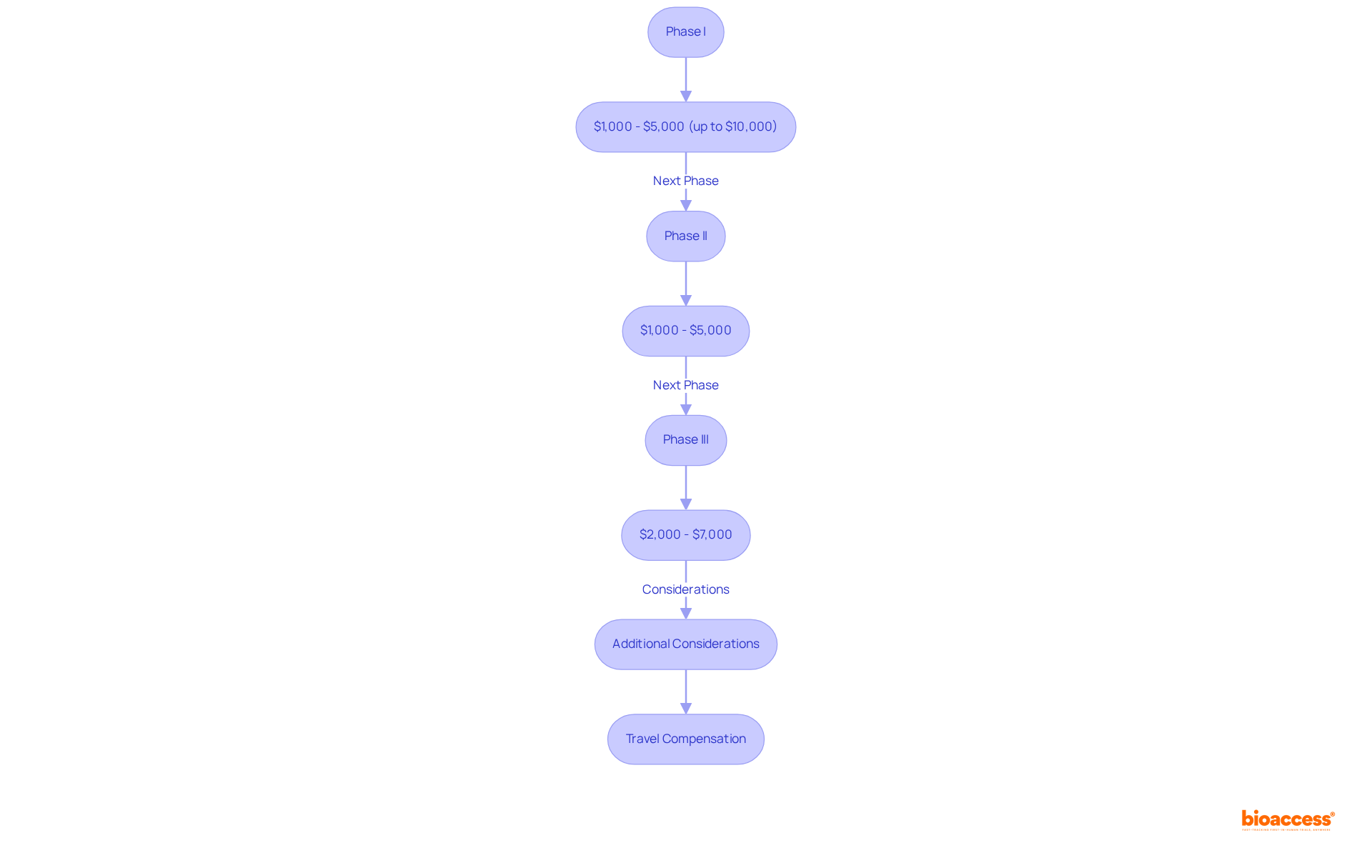
Compensation for participation in paid medical trials varies significantly based on the phase and specific requirements. In Phase I studies, which typically involve healthy volunteers, individuals can receive between $1,000 and $5,000, with some intensive research providing compensation exceeding $10,000. The median compensation for Phase I studies is approximately $3,070, reflecting the increased risks and time commitments associated with these early-phase studies.
As individuals progress to subsequent phases, such as Phase II and Phase III, compensation structures adjust accordingly. Phase II studies generally offer between $1,000 and $5,000, while Phase III studies typically provide compensation ranging from $2,000 to $7,000, influenced by factors such as study complexity and subject requirements.
Furthermore, research studies frequently compensate individuals for travel costs, which can enhance the financial attractiveness of participation. Understanding these compensation frameworks is crucial for prospective individuals, enabling them to make informed choices regarding their participation in a paid medical trial.
It is also advisable for individuals to consult tax advisors regarding their compensation to understand any financial obligations. Ethical considerations surrounding participant compensation must also be acknowledged, ensuring that payments reflect time and inconvenience rather than being perceived as buying consent.
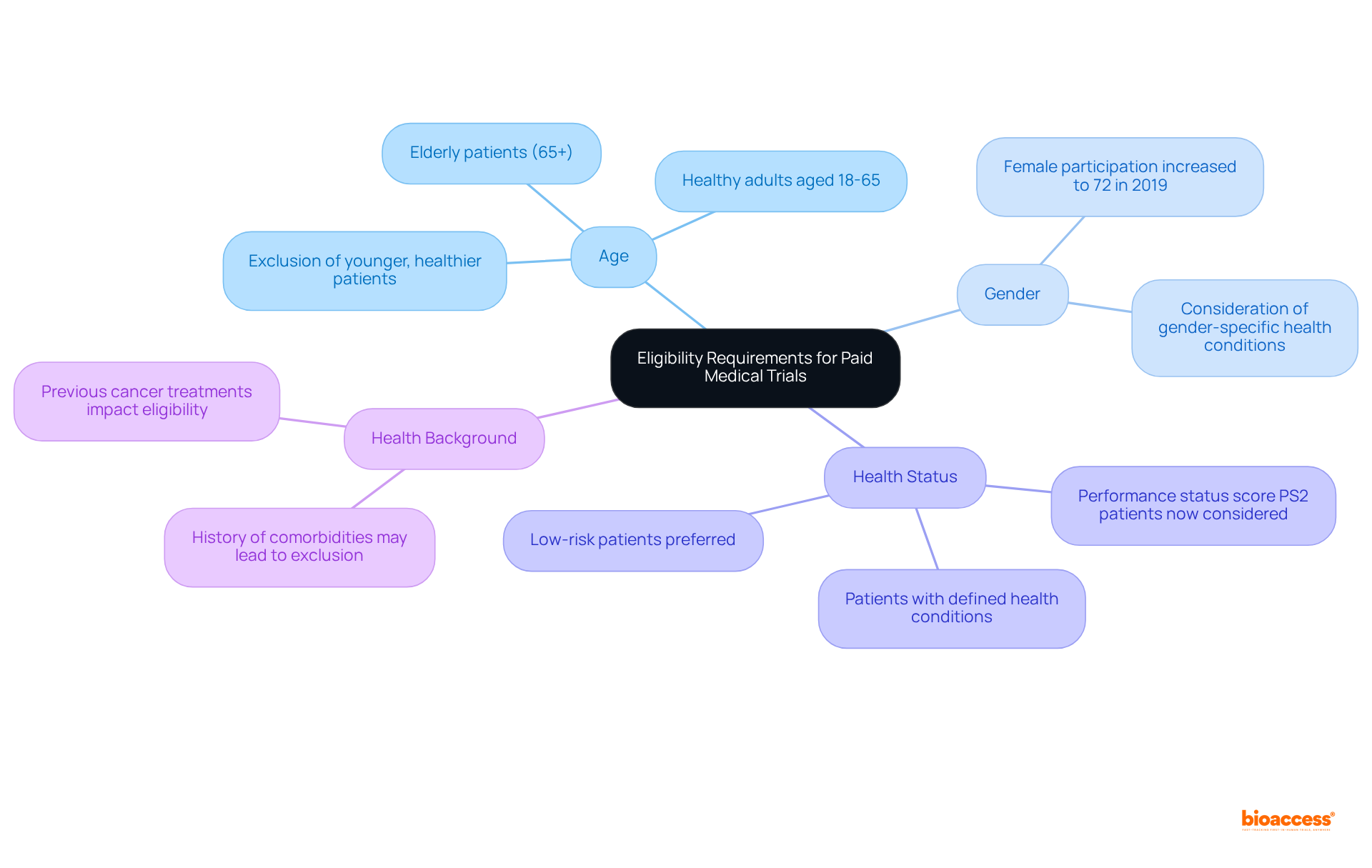
Eligibility criteria for compensated health studies vary significantly based on the research objectives. Common criteria encompass age, gender, health status, and health background. For example, certain studies may stipulate that subjects must be healthy adults aged 18-65, while others may specifically seek individuals with defined health conditions. It is essential for prospective candidates to meticulously evaluate these standards to determine their suitability prior to applying for a study.
Participating in a paid medical trial entails inherent dangers that are meticulously assessed and communicated to potential volunteers. These risks can vary significantly, ranging from minor side effects to more severe health issues, depending on the specific intervention under investigation. Prior to enrollment, individuals receive comprehensive information detailing potential risks, enabling them to make informed decisions by weighing the benefits against possible hazards.
Continuous monitoring during the experiment is crucial, as it facilitates the prompt identification and mitigation of risks, thereby safeguarding the welfare of participants. Notably, a thorough analysis of 475 studies involving 27,185 individuals revealed that while mild and moderate adverse events were common, the incidence of severe adverse events was strikingly low, with a median rate of zero per 1,000 individuals in the treatment group per day of observation. The overall rate of both associated and unassociated serious adverse occurrences was 0.025 per 100 individuals per day, further underscoring the safety of participants in research studies.
Furthermore, the median duration of adverse event monitoring was 16 days, demonstrating a commitment to ongoing risk assessment. This underscores the importance of robust risk communication strategies in medical research, ensuring that individuals are well-informed and supported throughout their involvement.
Additionally, bioaccess's extensive management services for research studies—including feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting—enhance the safety and efficiency of research. It is essential to recognize that approximately 80% of research studies, such as paid medical trials, face delays or terminations due to recruitment challenges, highlighting the critical role of effective risk communication in attracting subjects.
As Helene Quie emphasizes, managing risks entails ensuring that the benefits are substantial enough to justify those risks, thereby adding an ethical dimension to the discourse on risk assessment.
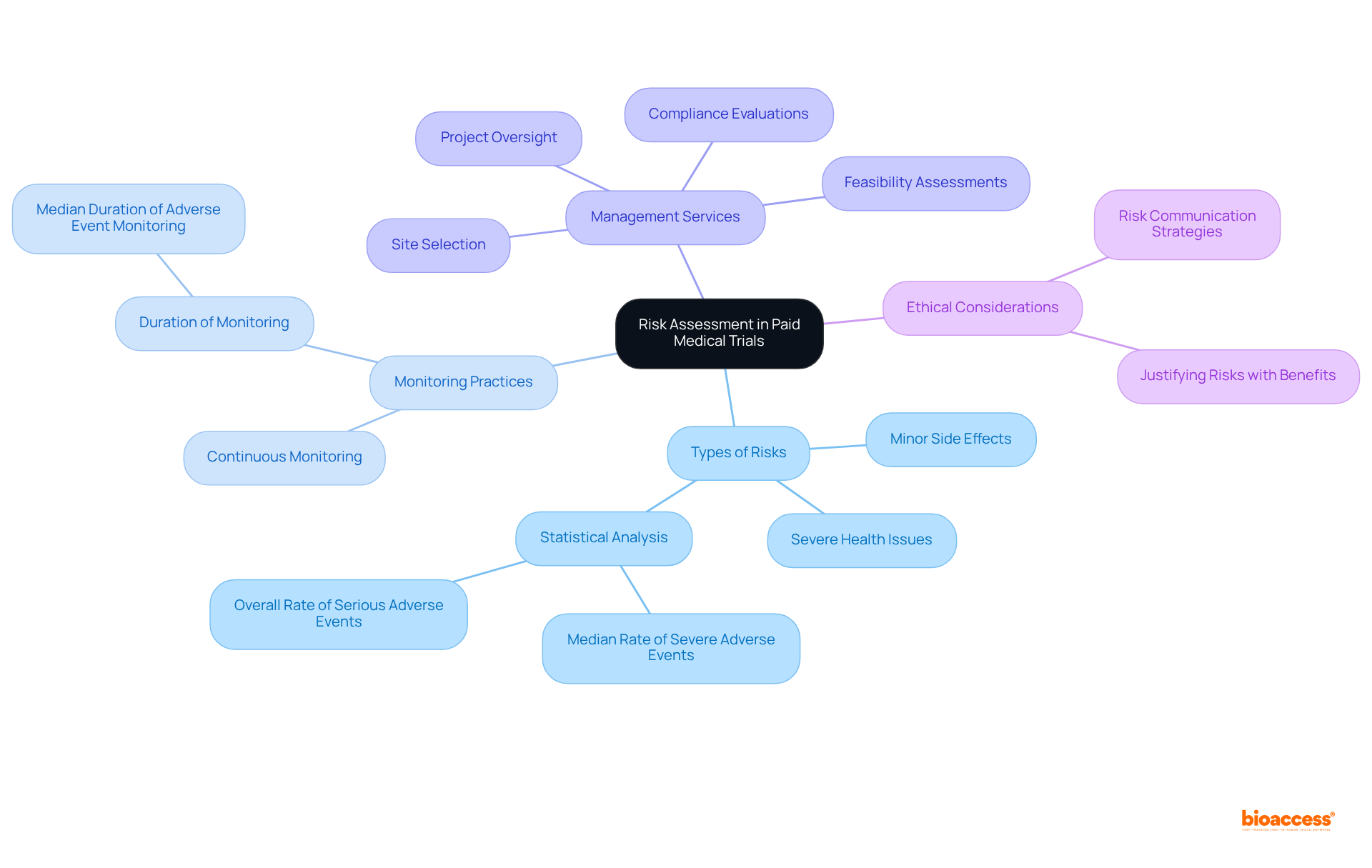
Informed consent stands as a cornerstone of clinical research, ensuring individuals possess a comprehensive understanding of their rights and the specifics of the study prior to participation. This essential process encompasses a clear grasp of the study's purpose, the procedures involved, potential risks, and the unequivocal right to withdraw at any moment without incurring penalties. Individuals must feel empowered to pose inquiries and seek clarification regarding any aspect of the trial, thereby fostering a culture of transparency and trust between researchers and participants.
Research indicates a significant variance in the understanding of informed consent elements among individuals. For instance, while an impressive 97.5% of participants recognized the importance of confidentiality, a mere 4.8% grasped the concept of placebo. This striking disparity highlights the urgent need for enhanced communication strategies throughout the informed consent process. Ethicists caution that the assumption of complete understanding can lead to ethical dilemmas in healthcare research, as many individuals may not fully comprehend the implications of their consent.
As discussions progress in 2025, the field underscores the necessity for improved reporting practices and transparency regarding consent rates, with studies revealing that approximately 26% of research efforts had consent rates falling below 70%. This situation calls for a thorough reassessment of how the rights of participants are communicated and understood in the context of paid medical trials. Key participant rights include:
All of which are vital for ethical healthcare practices and the promotion of shared decision-making.
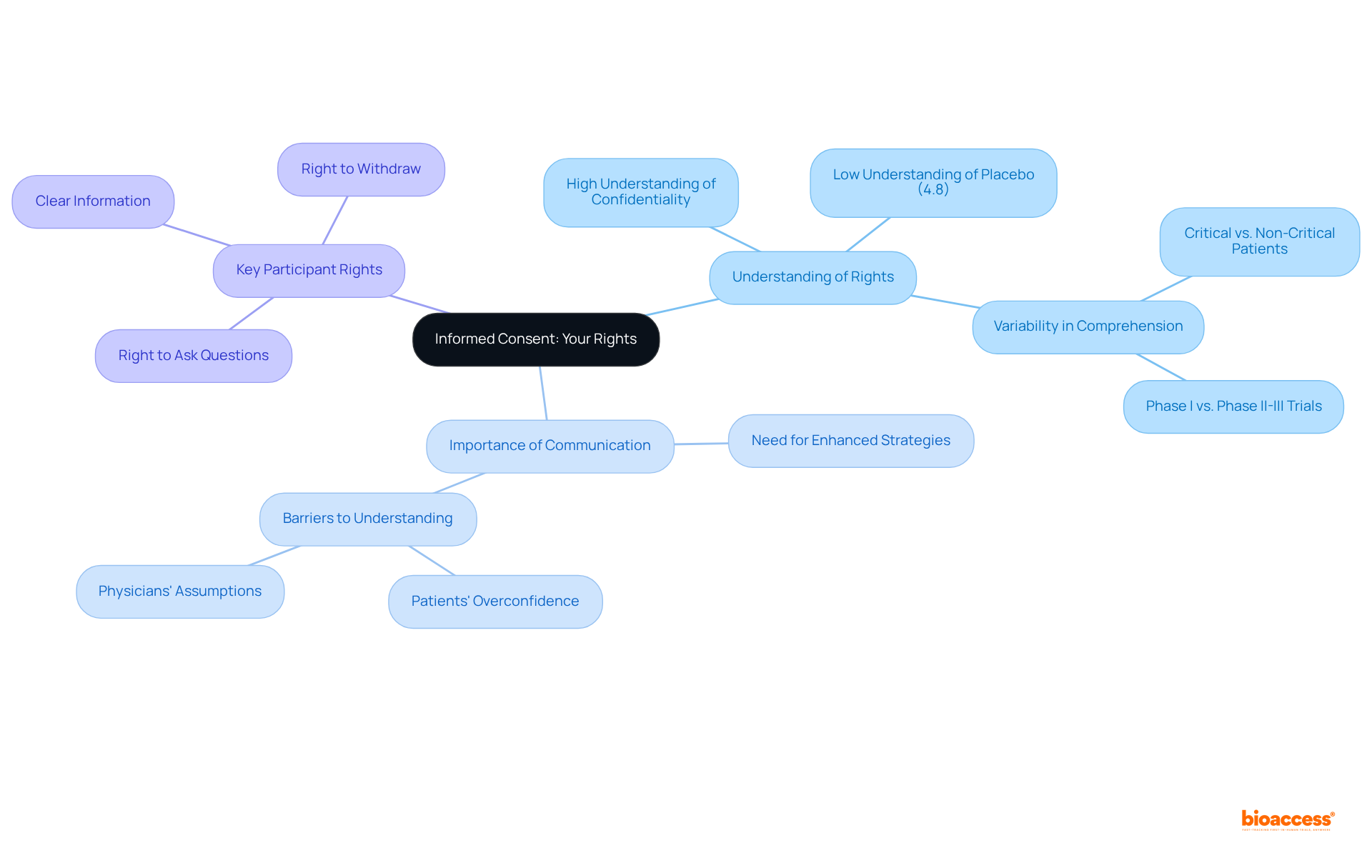
Paid medical trials encompass a diverse array of interventions, including new medications, healthcare devices, and therapeutic procedures. Notably, innovative medical devices such as implantable sensors and advanced prosthetics undergo rigorous assessment for their efficacy and safety within healthcare settings. Additionally, studies may evaluate the effectiveness of groundbreaking medications targeting specific conditions or investigate novel surgical techniques that promise improved patient outcomes.
Statistics reveal that cancer therapies dominate the clinical research landscape, accounting for 25% of all studies, followed closely by mental health and behavioral disorders at 15.1%. This trend emphasizes a significant commitment to developing innovative therapies that tackle urgent health challenges.
Researchers underscore the importance of aligning participation in a paid medical trial with individual health conditions and interests. Engaging in studies that resonate with personal health needs can enhance participant experiences and yield more meaningful results. As the realm of medical research continues to evolve, the integration of advanced technologies and personalized medicine is instrumental in shaping the types of interventions being tested, paving the way for future breakthroughs in healthcare.
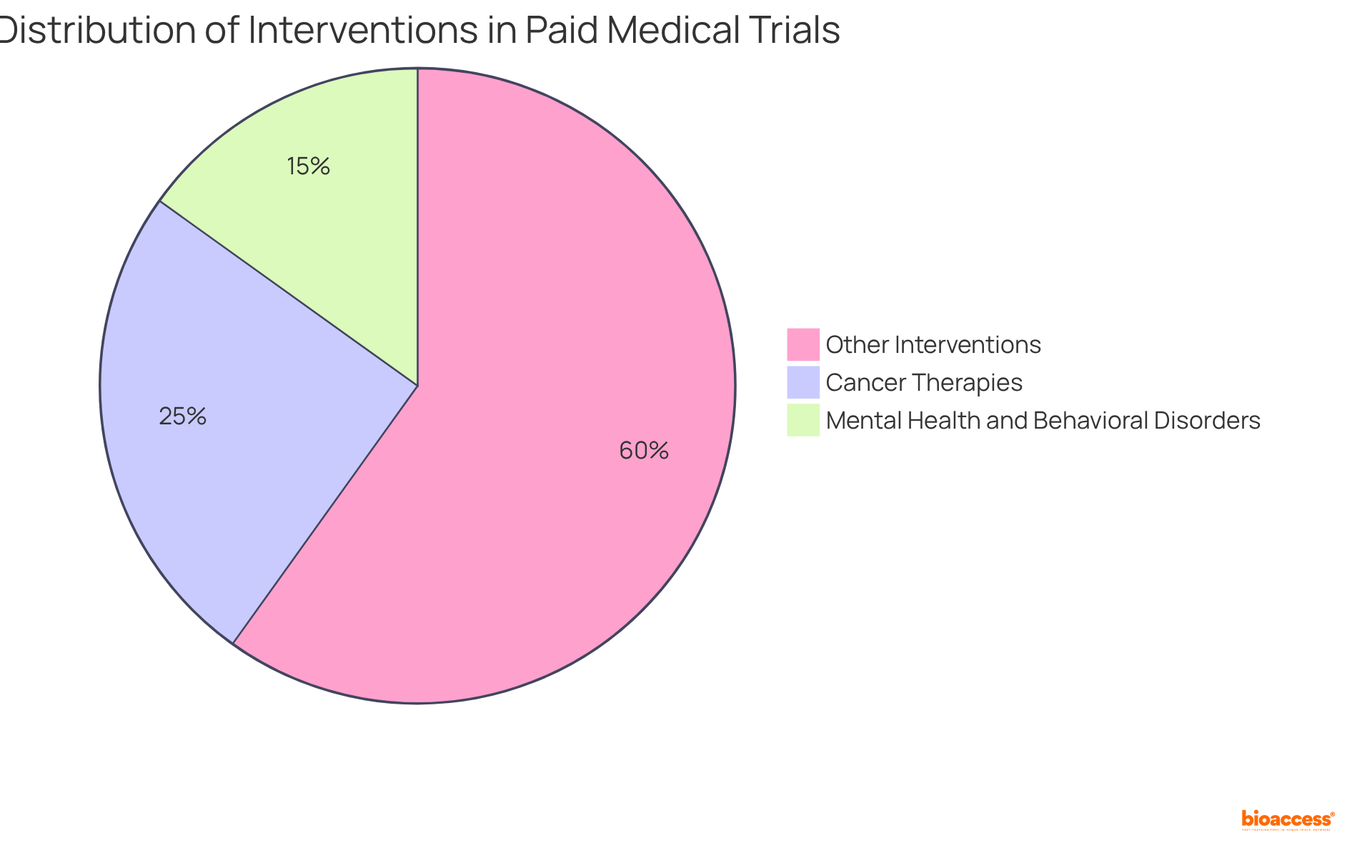
Clinical studies are meticulously organized into distinct stages, each designed to achieve specific objectives and provide unique experiences for participants. At bioaccess, we ensure that every phase is supported by comprehensive clinical study management services, enhancing the overall experience for both participants and stakeholders.
Understanding these stages equips individuals with insights into what to expect during their involvement, underscoring their vital role in advancing scientific research and enhancing patient support.
Regulatory oversight is paramount in ensuring that compensated medical studies adhere to ethical standards and safeguard participant rights. Organizations such as the FDA and Institutional Review Boards (IRBs) rigorously scrutinize study protocols to guarantee compliance with safety regulations and ethical guidelines. This oversight encompasses:
As a leading contract research organization (CRO) specializing in device studies in Latin America, bioaccess® plays a crucial role in navigating these regulatory environments, facilitating the introduction of innovative health technologies to the market while prioritizing the safety and rights of participants.
In 2025, the compliance rates with ethical standards in paid medical trials have shown notable improvement, reflecting a growing commitment to the well-being of participants. Regulatory agencies emphasize that ethical standards are not merely suggestions but fundamental principles that must be integrated into every aspect of research involving patients. These standards include:
By upholding these ethical standards, the integrity of research studies is preserved, fostering trust and encouraging participation. Furthermore, bioaccess®'s recent ACRP certification marks a significant milestone in enhancing clinical research standards in the region, further elevating the credibility and reliability of studies conducted under its auspices.
Identifying paid medical trials is crucial for advancing clinical research. To achieve this, various resources must be utilized effectively. Websites such as ClinicalTrials.gov provide comprehensive listings of ongoing studies, detailing eligibility requirements and contact information. Additionally, local hospitals, research institutions, and clinical research organizations like bioaccess® offer valuable insights into available studies, drawing on over 20 years of experience in managing:
Engaging with healthcare professionals is essential, as they can recommend studies that align with individual health conditions. This multifaceted approach not only aids participants in finding suitable trials but also fosters greater involvement in advancing medical research. By leveraging these resources and strategies, individuals can significantly enhance their chances of participating in a paid medical trial that contributes to impactful clinical research.
Participating in paid medical trials presents a unique opportunity to contribute to groundbreaking research while gaining access to innovative treatments. The insights shared throughout this article underscore the multifaceted aspects of these trials, from the efficiency of regulatory processes in regions like Latin America to the rigorous safety protocols that ensure participant well-being. Understanding the compensation structures, eligibility requirements, and the critical importance of informed consent empowers individuals to make informed decisions regarding their involvement in clinical research.
Key insights reveal that bioaccess® significantly accelerates the enrollment process, achieving rates that are 50% faster than traditional markets. The robust safety measures and continuous monitoring implemented in these trials highlight the unwavering commitment to participant protection. Moreover, the diverse types of interventions being tested—ranging from new medications to advanced medical devices—illustrate the vast potential for innovation in healthcare.
As the landscape of clinical research continues to evolve, the importance of participant engagement cannot be overstated. Individuals are encouraged to explore the resources available for finding suitable trials and to actively consider the benefits of participating in paid medical studies. By doing so, they not only contribute to the advancement of medical science but also potentially enhance their own health outcomes through access to cutting-edge therapies. Engaging in this vital area of research fosters a culture of collaboration that can lead to significant breakthroughs in healthcare for all.
What is bioaccess® and how does it enhance paid medical trials?
bioaccess® leverages the regulatory efficiency in Latin America, particularly Colombia, to reduce study costs by over 30% compared to North America and Western Europe. It also benefits from diverse patient demographics in the Balkans and streamlined ethical approvals in Australia, allowing for faster clinical studies and quicker participant enrollment.
How quickly can ethical approvals be obtained in Colombia and Australia?
In Colombia, ethical approvals can be secured in just 4-6 weeks. In Australia, public hospitals typically take 8-12 weeks for approvals, while private Human Research Ethics Committees can expedite the process to as little as 1-2 weeks.
What are the enrollment rates for bioaccess® compared to traditional markets?
bioaccess® achieves enrollment rates that are 50% faster than those in traditional markets, highlighting its competitive advantage in conducting paid medical trials.
What safety protocols are in place for participants in paid medical trials?
Safety protocols include comprehensive screening, continuous health monitoring, and strict adherence to regulatory guidelines. Institutional Review Boards (IRBs) oversee these studies to ensure safety measures are implemented and participants are informed of potential risks.
How does bioaccess® ensure participant safety during trials?
bioaccess® conducts regular safety audits, risk evaluations, and maintains high compliance rates with safety regulations. They also establish protocol-specified stopping rules to suspend studies if there are excessive safety incidents.
What compensation can participants expect from paid medical trials?
Compensation varies by study phase: Phase I studies typically offer $1,000 to $5,000 (with some exceeding $10,000), Phase II studies range from $1,000 to $5,000, and Phase III studies provide between $2,000 and $7,000. Participants may also be reimbursed for travel costs.
What ethical considerations surround compensation for trial participants?
It is important that compensation reflects participants' time and inconvenience rather than being seen as buying consent. Individuals are also advised to consult tax advisors regarding their compensation to understand any financial obligations.