


Effective trial risk management stands as a cornerstone of successful clinical studies, especially within the ALMBIH framework. By proactively identifying and addressing potential risks, organizations not only safeguard participant safety but also enhance the overall efficacy of their trials. Yet, the challenge lies in navigating the complexities of risk assessment and response strategies in a landscape that continually evolves with new technologies.
How can clinical research teams leverage these advancements to identify threats effectively? Moreover, how can they implement robust mitigation strategies that ensure compliance and improve outcomes? These questions are crucial as they guide the development of effective risk management practices that are essential for the success of clinical trials.
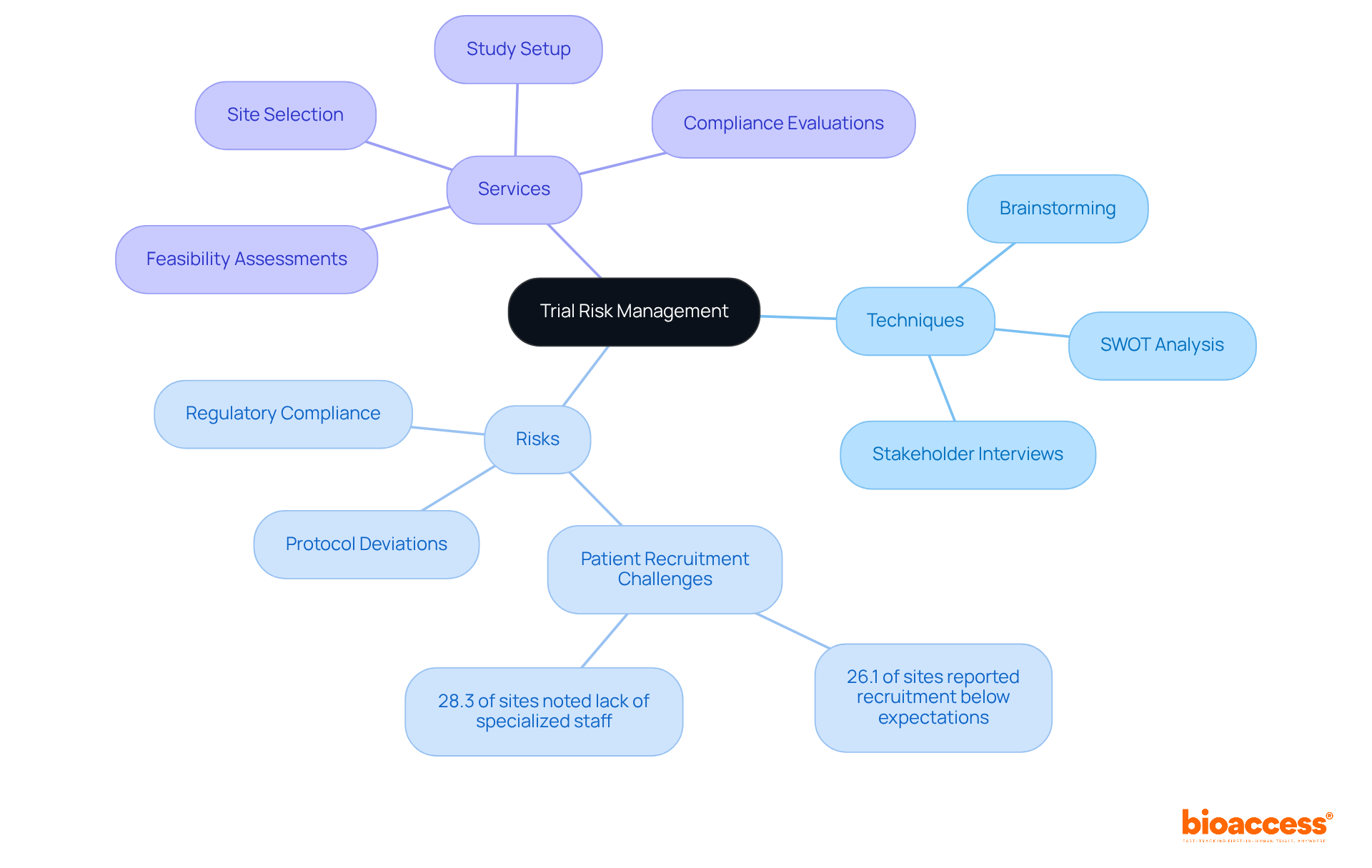
Effectively managing uncertainties in clinical studies is paramount for trial risk management planning under almbih. Recognizing potential dangers early on can significantly enhance trial outcomes and contribute to effective trial risk management planning under almbih, thereby improving participant safety. This requires thorough pre-hearing evaluations and continuous monitoring of the assessment environment. Techniques such as brainstorming sessions, SWOT analysis, and stakeholder interviews are invaluable for uncovering uncertainties related to trial risk management planning under almbih, including:
For instance, bioaccess™ offers comprehensive clinical study management services, including:
These services are essential for early issue detection. A study conducted in Bosnia and Herzegovina underscored the importance of early hazard identification, revealing that:
By cultivating a culture of vigilance and encouraging team members to report potential threats, organizations can adopt trial risk management planning under almbih to minimize the risk of adverse events.
Moreover, leveraging systems like the NIH Clinical Center's electronic occurrence reporting system, which documents over 5,000 reports annually, highlights the critical role of ongoing monitoring in effective management. The partnership between bioaccess™ and Caribbean Health Group, supported by Colombia's Minister of Health, exemplifies a commitment to enhancing clinical trial environments in Barranquilla. This collaboration positions Barranquilla as a leading destination for clinical research in Latin America.
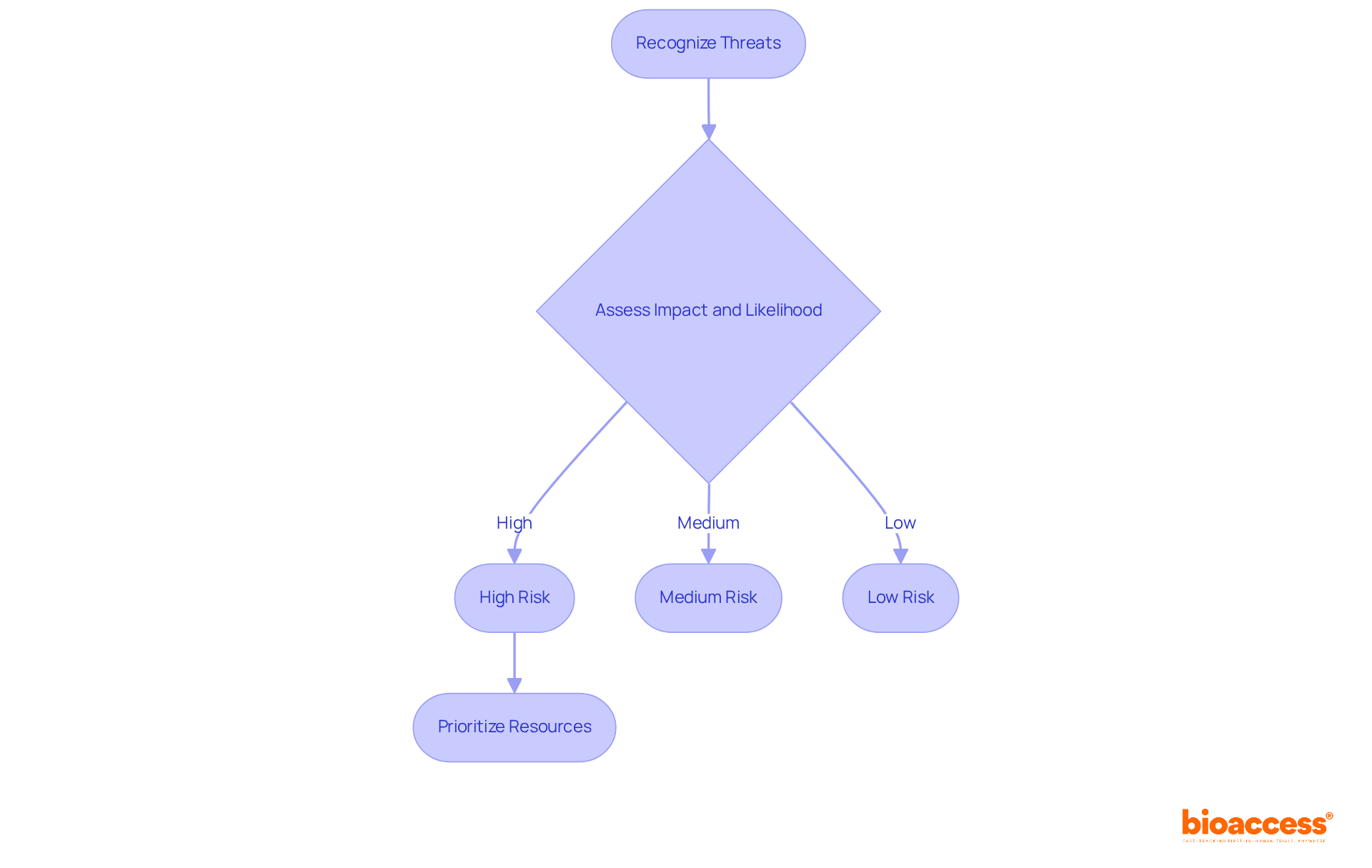
Recognizing threats is just the beginning; the next crucial step is to assess them based on their potential impact and likelihood of occurrence. This assessment can be conducted using both qualitative and quantitative methods, such as matrices and scoring systems.
For instance, threats that pose a significant risk to patient safety must be prioritized over those that might only cause minor delays. By categorizing hazards into high, medium, and low levels, healthcare research teams can allocate resources more effectively and devise targeted mitigation strategies.
A compelling case study from a recent clinical trial in the Balkans demonstrated that a systematic analysis of uncertainties not only facilitated the navigation of regulatory hurdles but also significantly enhanced the efficiency of the experiment. This underscores the tangible benefits of robust uncertainty assessment methods in clinical research.
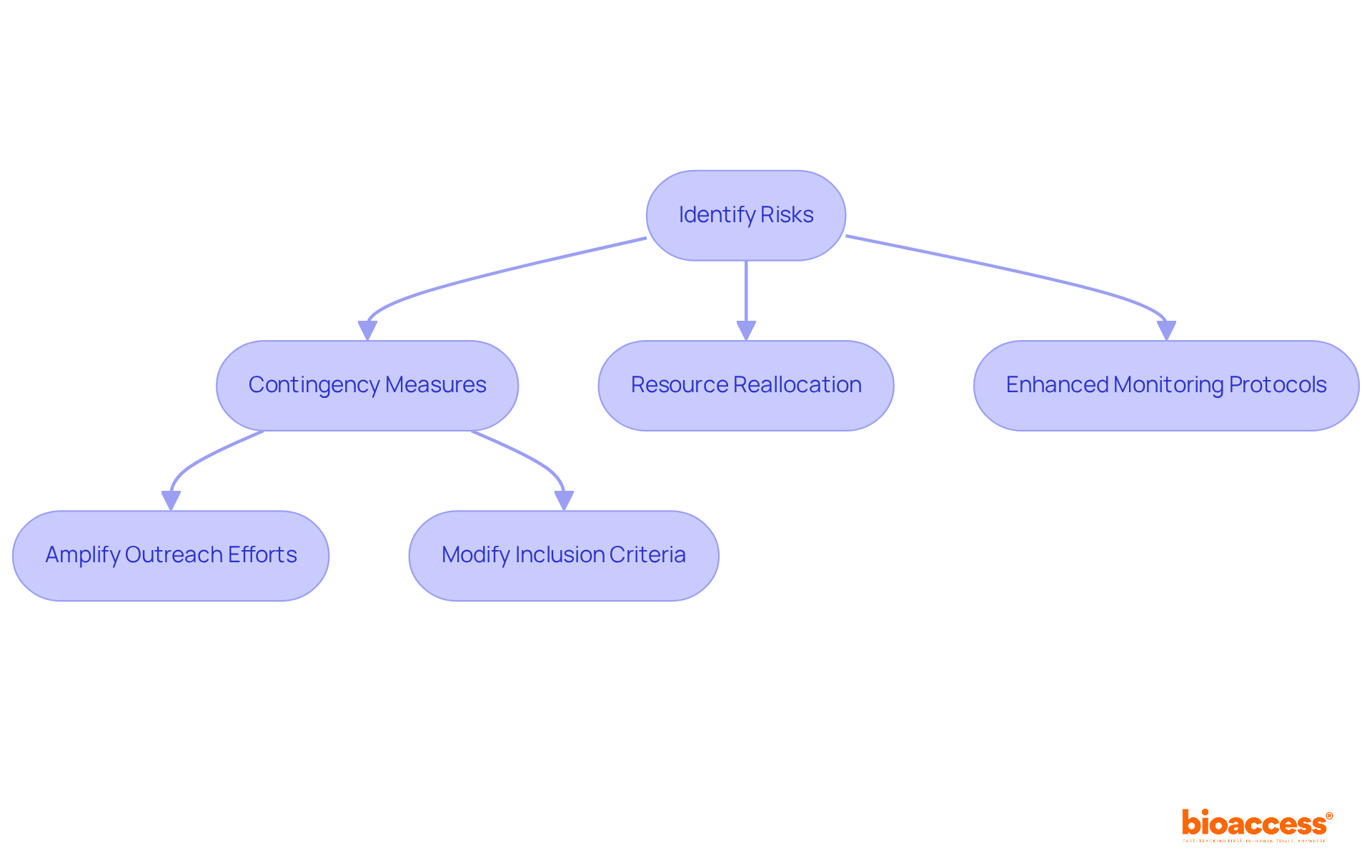
Developing efficient response strategies for identified threats is crucial in clinical research, necessitating trial risk management planning under almbih to outline specific actions for mitigating each threat. This plan may include:
For instance, when faced with challenges in patient recruitment, strategies could involve amplifying outreach efforts or modifying inclusion criteria to broaden eligibility.
A significant example of effective strategy execution occurred in a recent study with GlobalCare Clinical Trials. Proactive adjustments made in partnership with bioaccess led to an impressive 50% decrease in recruitment time and a remarkable 95% retention rate. Such outcomes underscore the importance of having a well-defined strategy in place.
By ensuring that all team members understand their responsibilities in response measures, organizations can cultivate a collaborative atmosphere that significantly enhances the robustness of healthcare studies. This collaboration not only addresses immediate challenges but also sets the stage for future successes in clinical research.
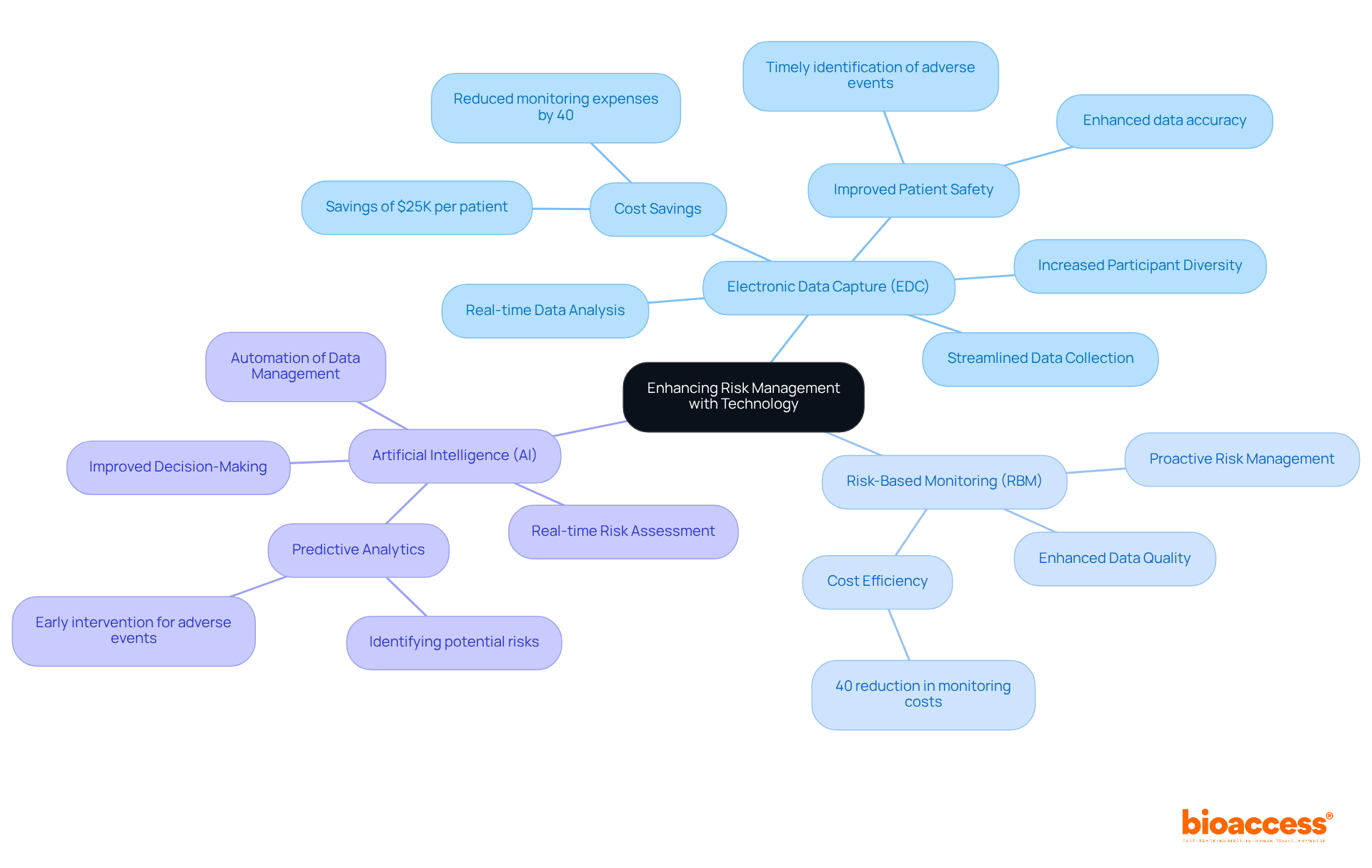
Incorporating advanced technology into management practices significantly enhances the efficiency and effectiveness of clinical studies. Electronic data capture (EDC) systems, risk-based monitoring (RBM) platforms, and artificial intelligence (AI) are pivotal in enabling real-time data analysis and assessment. For example, bioaccess accelerates the enrollment of treatment-naive cardiology or neurology cohorts by 50% compared to Western sites, achieving savings of $25K per patient with FDA-ready data-no rework, no delays. AI algorithms can analyze past study data to predict potential risks, allowing teams to implement proactive measures.
To improve safety oversight practices, it’s crucial to define measurable threat elements associated with the study, such as dropout rates and adverse events. A notable case involved the implementation of an RBM system in a research study in Australia, which resulted in a 40% reduction in monitoring expenses while simultaneously enhancing data quality. By adopting these technological advancements, organizations can improve their trial risk management planning under almbih, streamline their risk oversight processes, enhance patient safety, and ensure compliance with regulatory standards.
Moreover, bioaccess offers comprehensive research study management services, including:
The anticipated growth of the AI-driven medical trials market, projected to reach $21.79 billion by 2030, underscores the importance of embracing these technologies. EDC systems not only improve data accuracy and efficiency but also bolster data security and facilitate real-time information sharing, ultimately leading to more successful clinical outcomes.
Ongoing data analysis of Key Risk Indicators (KRIs) is essential to identify trends and deviations that indicate rising risks, further solidifying the role of EDC in enhancing participant recruitment and enrollment.
Effective trial risk management planning under ALMBIH is crucial for enhancing clinical study outcomes and ensuring participant safety. By identifying risks early, analyzing their potential impact, crafting response strategies, and leveraging technology, organizations can navigate uncertainties more effectively. This comprehensive approach minimizes adverse events and fosters a culture of vigilance and collaboration among team members.
The article highlights several key practices, including:
These insights underscore the critical nature of proactive risk management in achieving successful trial outcomes.
Ultimately, embracing these best practices in trial risk management is not merely a strategic advantage; it is a vital commitment to safeguarding participant welfare and ensuring the integrity of clinical research. By prioritizing early risk identification and utilizing innovative technology, organizations can position themselves as leaders in the evolving landscape of clinical trials, paving the way for more effective and safer healthcare solutions.
Why is early identification of risks important in clinical studies?
Early identification of risks is crucial as it significantly enhances trial outcomes and contributes to effective trial risk management planning, ultimately improving participant safety.
What techniques are recommended for uncovering uncertainties in trial risk management?
Recommended techniques include brainstorming sessions, SWOT analysis, and stakeholder interviews.
What are some common uncertainties related to trial risk management?
Common uncertainties include protocol deviations, patient recruitment challenges, and regulatory compliance issues.
What services does bioaccess™ provide for clinical study management?
Bioaccess™ offers services such as feasibility assessments, site selection, study setup, and compliance evaluations, which are essential for early issue detection.
What findings were revealed by a study conducted in Bosnia and Herzegovina regarding trial risks?
The study found that 28.3% of sites reported a shortage of specialized staff as a concern, and 26.1% faced recruitment challenges below expectations.
How can organizations cultivate a culture of vigilance in trial risk management?
Organizations can cultivate a culture of vigilance by encouraging team members to report potential threats and adopting proactive trial risk management strategies.
What role does ongoing monitoring play in trial risk management?
Ongoing monitoring is critical for effective management, as it helps document and address issues as they arise, exemplified by systems like the NIH Clinical Center's electronic occurrence reporting system.
What is the significance of the partnership between bioaccess™ and Caribbean Health Group?
This partnership, supported by Colombia's Minister of Health, aims to enhance clinical trial environments in Barranquilla, positioning it as a leading destination for clinical research in Latin America.