


The biopharmaceutical industry stands at a pivotal moment, poised for transformation as it strives to streamline clinical trial processes and enhance patient access to groundbreaking therapies. By implementing effective strategies, organizations can significantly reduce activation timelines for trial sites, leading to faster recruitment and improved study outcomes.
However, the path is fraught with challenges, including:
What best practices can biopharma companies adopt to overcome these hurdles and ensure timely and efficient trial site activation?
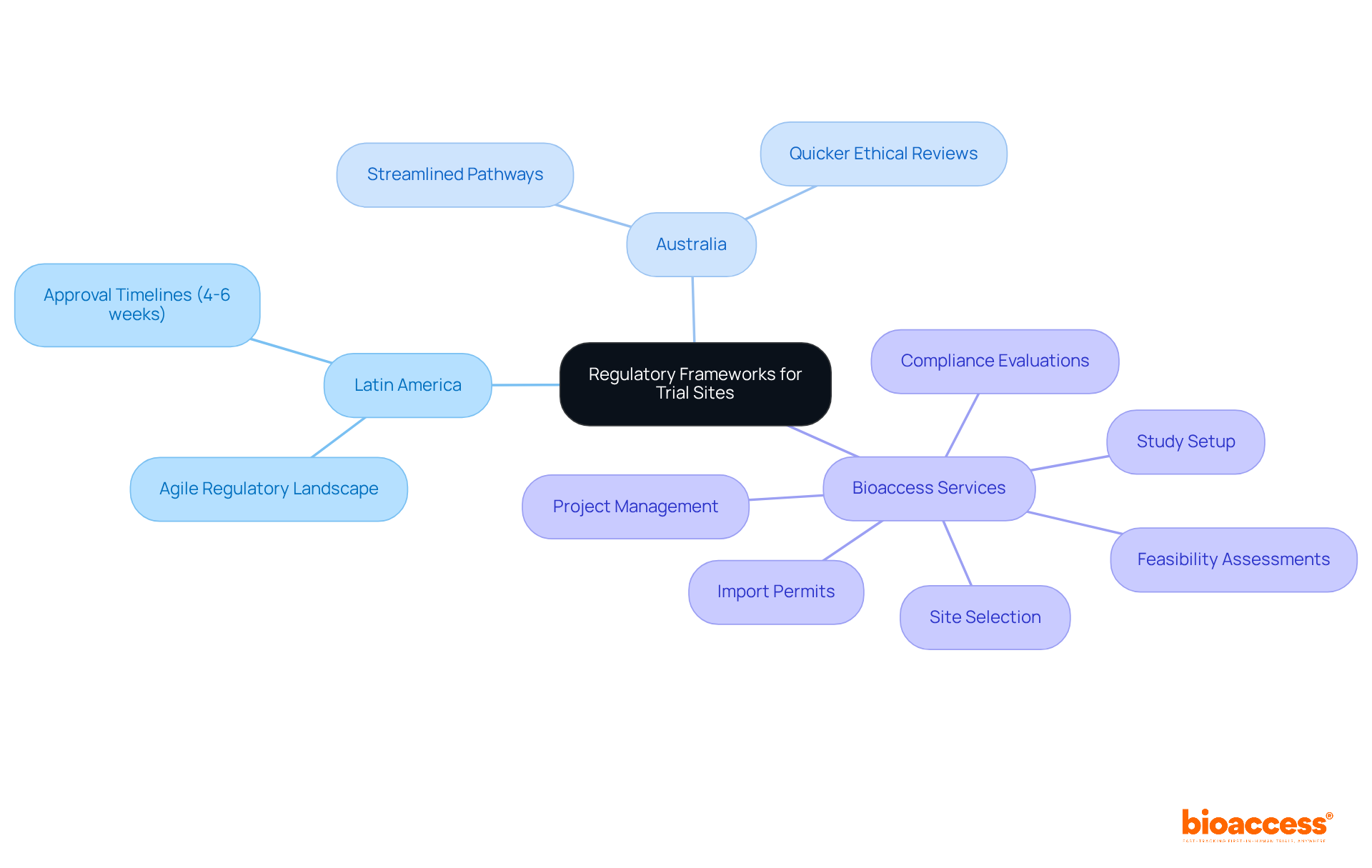
To enhance biopharma study activation timelines, understanding the regulatory frameworks governing clinical studies is essential. Each region presents distinct regulations that can significantly influence the speed of study initiation. For instance, in Latin America, the regulatory landscape is notably agile, permitting ethical approvals in as little as 4-6 weeks, which can substantially reduce overall timelines. Likewise, Australia provides streamlined pathways that enable quicker ethical reviews, further accelerating the process.
Familiarizing yourself with these regulations not only aids in effective planning but also helps you sidestep common pitfalls that can cause delays, such as incomplete documentation or miscommunication with regulatory bodies. Moreover, bioaccess's comprehensive clinical study management services - including feasibility assessments, site selection, compliance evaluations, study setup, import permits, nationalization of investigational devices, and project management - ensure that all necessary documentation is meticulously prepared and submitted on time.
Maintaining open lines of communication with regulatory bodies can clarify processes and expedite approvals. Regulatory specialists have noted that proactive engagement with health agencies can significantly enhance the biopharma trial site activation timelines under alims, ultimately leading to faster access for individuals to innovative therapies. By leveraging bioaccess's expertise, you can navigate the complexities of clinical research with confidence.
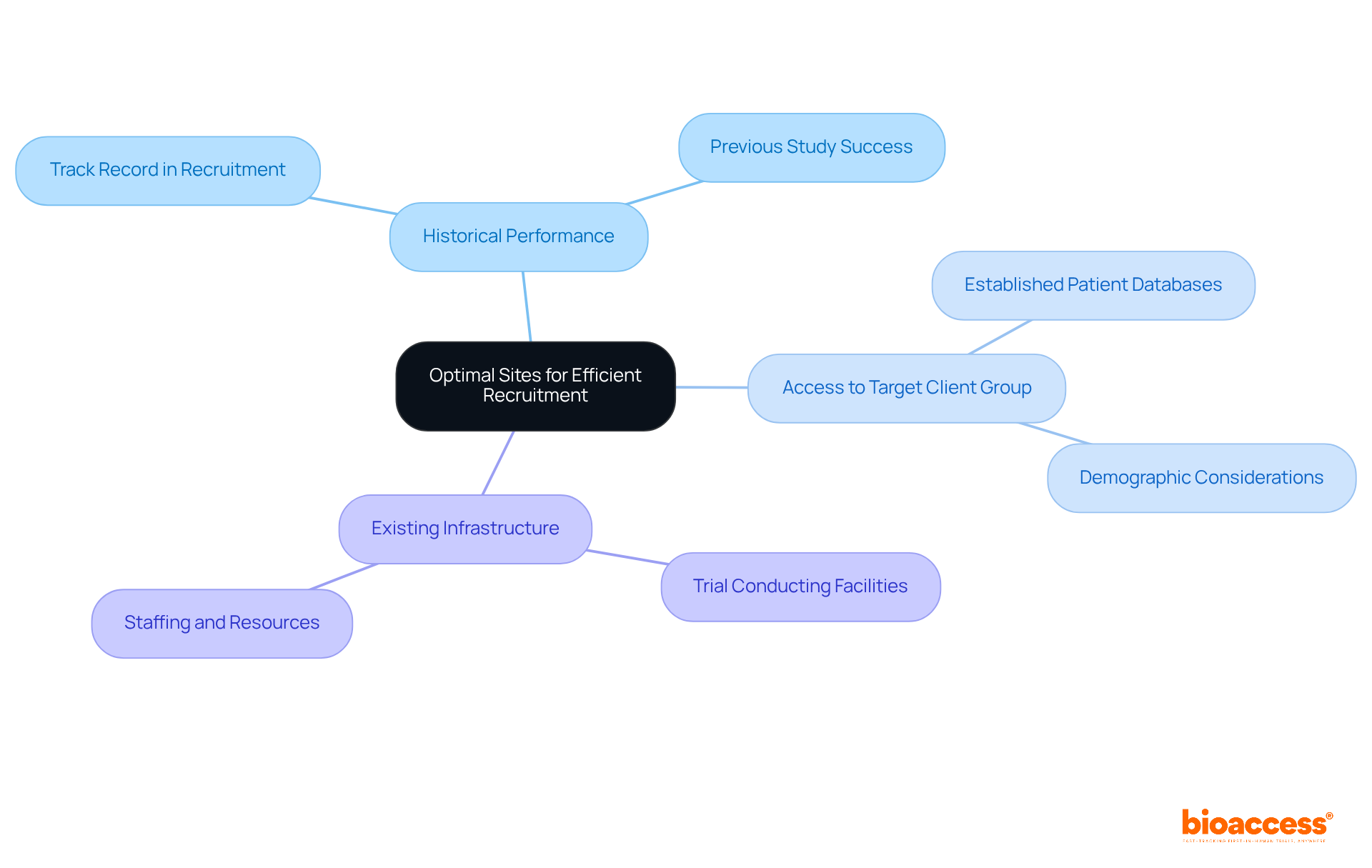
Choosing the best clinical research locations is crucial for enhancing recruitment effectiveness and minimizing biopharma trial site activation timelines under alims. Key factors to consider include:
Feasibility studies are vital in pinpointing locations that not only meet the study's criteria but also demonstrate a strong track record in participant recruitment. Involving platforms early in the planning phase fosters better alignment of expectations and resources, leading to a more streamlined activation process.
For instance, locations with established patient databases or prior experience with similar studies can significantly expedite participant enrollment, ultimately boosting overall study efficiency. Recent trends indicate that leveraging AI-driven location selection can enhance the identification of top-enrolling sites by 30 to 50 percent, further accelerating recruitment by 10 to 15 percent across various therapeutic areas. This strategic approach not only optimizes timelines but also elevates the quality and diversity of clinical studies.
With bioaccess®, you can achieve enrollment 50% faster than Western sites, translating to $25K savings per individual through FDA-ready data-no rework, no delays. This capability is exemplified by bioaccess's collaboration with Welwaze Medical Inc. for the launch of the Celbrea® medical device in Colombia, showcasing how strategic partnerships can enhance recruitment efficiency and regulatory access.
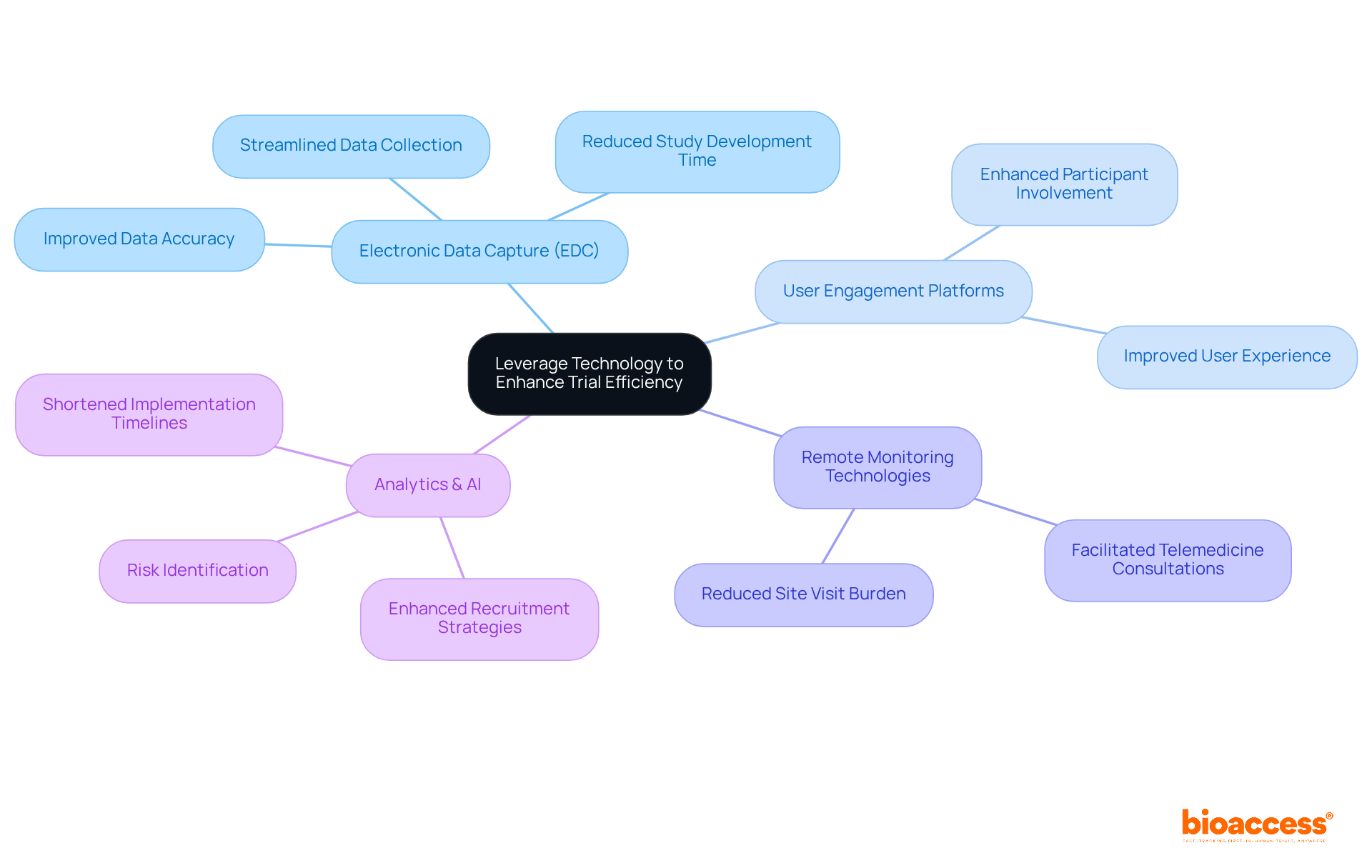
Incorporating technology into clinical research processes significantly enhances efficiency and data accuracy. Electronic data capture (EDC) systems, user engagement platforms, and remote monitoring technologies streamline data collection and enhance participant involvement. For instance, telemedicine enables remote consultations, allowing patients to participate in studies without the hassle of frequent site visits.
Moreover, utilizing analytics and artificial intelligence can pinpoint potential risks and enhance recruitment strategies, ultimately shortening implementation timelines. By adopting these technological advancements, study sponsors can improve overall outcomes and accelerate the path to bringing innovative therapies to market.
The Medtech landscape is evolving rapidly, and organizations must adapt to these changes to address key challenges effectively. Collaboration among stakeholders is essential to leverage these technologies fully. As we move forward, it’s crucial to consider how these advancements can be integrated into existing frameworks to optimize clinical research processes.
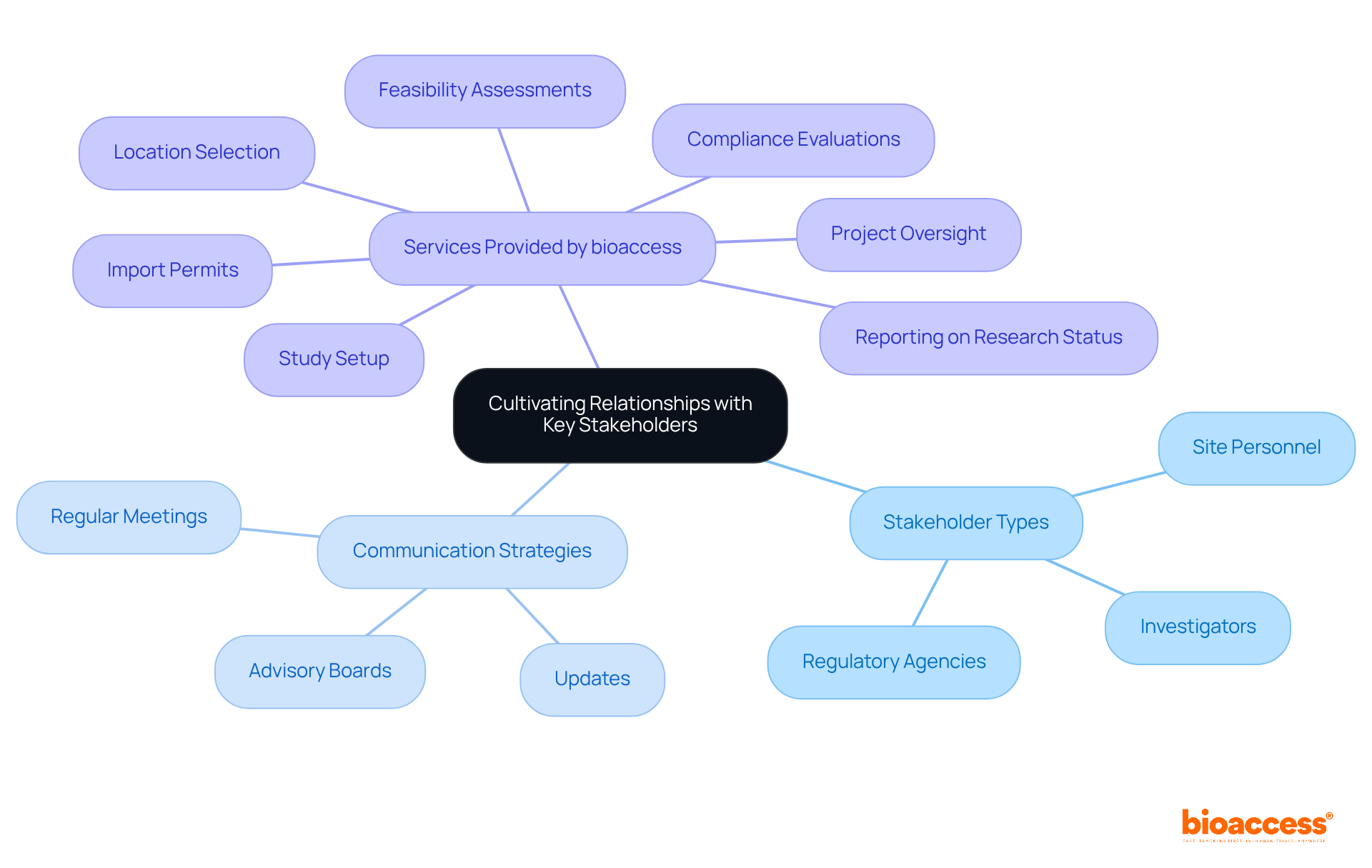
Establishing and cultivating strong connections with key stakeholders - such as investigators, regulatory agencies, and site personnel - is vital for successful study activation. Effective communication builds trust, which in turn improves collaboration and problem-solving throughout the process. Regular meetings and updates are essential for aligning expectations and proactively addressing concerns. Engaging stakeholders early in the planning phases fosters a sense of ownership and dedication to the project's success. For instance, the establishment of advisory boards or steering committees can facilitate ongoing dialogue, ensuring that all parties remain engaged and informed. This cooperative method not only optimizes testing procedures but also greatly enhances biopharma trial site activation timelines under alims, as demonstrated by successful collaborations in recent biopharma studies.
bioaccess® has activated over 50 pre-qualified locations in less than eight weeks, demonstrating its commitment to accelerated patient recruitment and location activation services. The extensive clinical study management services provided by bioaccess encompass:
According to a survey, 92% of respondents identified contract and budget delays as top areas for improvement, underscoring the necessity of effective communication in overcoming these challenges. As Brian Mallon stated, "By simplifying documentation, smoothing negotiations, and fostering open collaboration, we can build a thriving ecosystem for trial start-ups - one that prioritizes site needs and accelerates access to new therapies.
Enhancing biopharma trial site activation timelines is a complex challenge that demands a thorough grasp of regulatory frameworks, strategic site selection, technological integration, and strong stakeholder relationships. By adopting these best practices, organizations can significantly streamline the activation process, ensuring that innovative therapies reach patients more swiftly and efficiently.
Key insights from this discussion underscore the necessity of navigating regulatory landscapes, which differ by region and can either accelerate or impede trial timelines. Moreover, selecting optimal sites based on historical performance and patient access is crucial for efficient recruitment. Utilizing technology, such as electronic data capture and telemedicine, further boosts the speed and accuracy of clinical trials. Lastly, nurturing robust relationships with stakeholders fosters effective communication, collaboration, and alignment of expectations throughout the study lifecycle.
In summary, the future of biopharma trial site activation relies on the implementation of these best practices. By prioritizing regulatory understanding, optimizing site selection, embracing technology, and cultivating stakeholder relationships, organizations can enhance activation timelines and contribute to a more efficient clinical trial landscape. Adopting these strategies will ultimately lead to improved patient access to groundbreaking therapies and a more agile biopharma industry.
Why is it important to understand regulatory frameworks for trial sites?
Understanding regulatory frameworks is essential to enhance biopharma study activation timelines, as each region has distinct regulations that can significantly influence the speed of study initiation.
How long does it typically take to obtain ethical approvals in Latin America?
In Latin America, the regulatory landscape allows for ethical approvals in as little as 4-6 weeks, which can substantially reduce overall study timelines.
What advantages does Australia offer regarding ethical reviews for clinical studies?
Australia provides streamlined pathways that enable quicker ethical reviews, further accelerating the study initiation process.
What common pitfalls can cause delays in clinical studies?
Common pitfalls include incomplete documentation and miscommunication with regulatory bodies, which can lead to delays in study activation.
What services does bioaccess offer to assist with clinical study management?
Bioaccess offers comprehensive clinical study management services, including feasibility assessments, site selection, compliance evaluations, study setup, import permits, nationalization of investigational devices, and project management.
How can maintaining communication with regulatory bodies benefit trial sites?
Maintaining open lines of communication with regulatory bodies can clarify processes and expedite approvals, ultimately enhancing trial site activation timelines.
What role do regulatory specialists play in the process of clinical research?
Regulatory specialists can provide proactive engagement with health agencies, which can significantly enhance biopharma trial site activation timelines and lead to faster access to innovative therapies.