


To find clinical trials that offer compensation for participation, individuals must utilize reputable registries, clearly specify their location and eligibility criteria, and actively search for studies that outline compensation details. This article outlines the essential steps necessary to identify and enroll in such trials. It emphasizes the critical importance of understanding compensation structures, eligibility requirements, and the informed consent process. By doing so, participants can ensure a safe and informed experience throughout their involvement in clinical research.
Navigating the world of clinical trials presents an exciting yet daunting challenge, particularly when considering the potential for compensation. With over 64,000 active studies actively seeking participants, a significant opportunity exists for individuals to contribute to groundbreaking medical research while earning financial rewards. However, the journey of finding and enrolling in these trials often involves complexity and uncertainty.
How can one effectively identify clinical trials that align with personal health goals and offer payment for participation? This guide unveils a step-by-step approach to uncovering paid clinical trials, ensuring informed decisions and maximizing potential benefits.
Clinical studies are meticulously structured investigations involving human participants, designed to evaluate the safety and efficacy of new healthcare interventions, including drugs, devices, and treatment protocols. These experiments are crucial in generating information that can lead to groundbreaking treatments or enhance existing therapies, thereby advancing health knowledge.
As of 2025, there are over 452,000 registered studies worldwide, with 64,838 actively seeking participants, showcasing a strong commitment to research and progress in healthcare. Successful studies have historically paved the way for innovative treatments, underscoring their importance in the healthcare landscape.
Specialists emphasize that these research studies are conducted under rigorous ethical and safety guidelines, ensuring participant protection while striving to improve healthcare outcomes. The stringent procedures followed in these studies are vital for validating new therapies before they become widely available, establishing these investigations as a cornerstone of modern medical research.
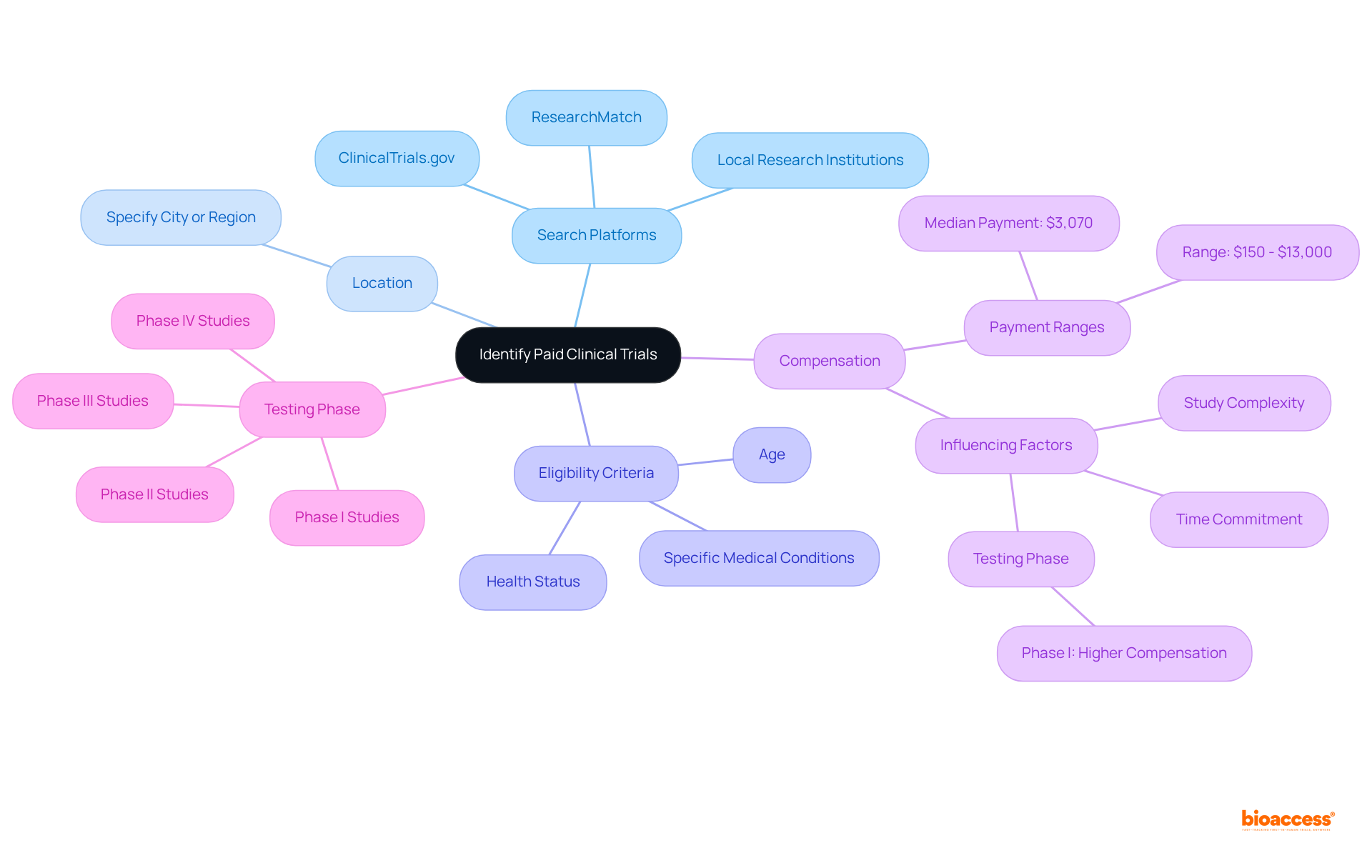
To find clinical trials that pay, start by exploring reputable clinical study registries such as ClinicalTrials.gov, ResearchMatch, and local research institutions. When conducting your search, consider the following criteria:
Utilize terms like 'clinical trials that pay' or 'compensation for involvement' in your searches to enhance your results. Additionally, consider registering for notifications from specific research platforms such as ResearchMatch or ClinicalTrials.gov to stay updated on new opportunities. This proactive approach can significantly improve your chances of discovering suitable paid opportunities that align with your interests and qualifications.
It is also essential to consider the ethical implications of compensation in research studies, ensuring that participants are treated with respect and dignity while being adequately informed about the potential risks and benefits.
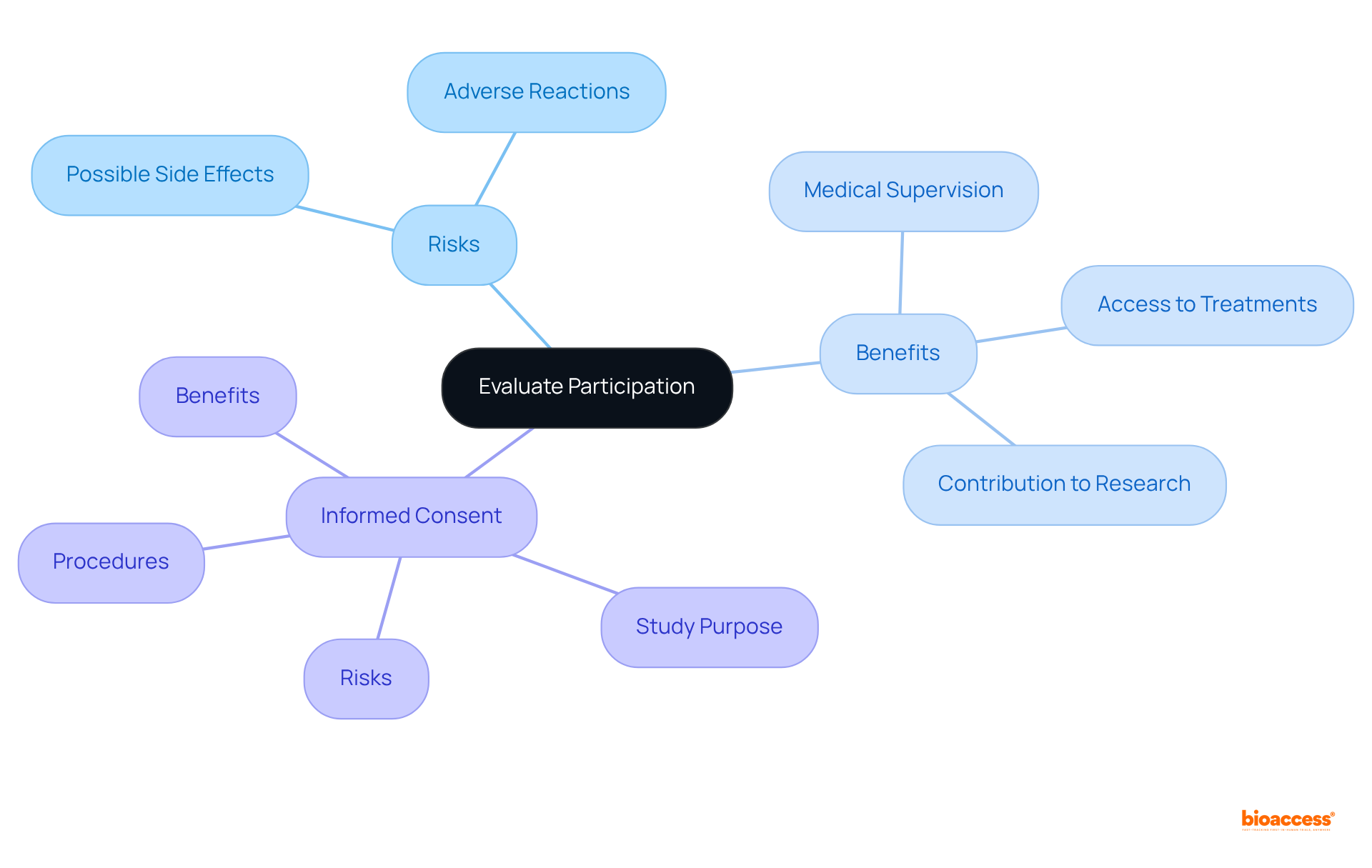
Before joining a clinical study, it is essential to assess the potential risks and benefits. Understanding these factors is crucial for making an informed decision about participation.
Risks: It is vital to comprehend the possible side effects or adverse reactions associated with the treatment being tested. Research indicates that a significant percentage of participants experience side effects, which should be clearly outlined in the informed consent document.
Benefits: Consider the potential advantages, such as access to innovative treatments, close medical supervision, and the opportunity to contribute to medical research that may benefit others in the future.
Informed Consent: This process educates participants about the study's purpose, procedures, risks, and benefits. Thoroughly reading and comprehending the informed consent form is vital before agreeing to participate. If any aspect is unclear, do not hesitate to ask questions.
Taking the time to evaluate these factors empowers you to make a knowledgeable choice about involvement in a research study, ensuring that you understand both the ethical considerations and the possible effects on your health.
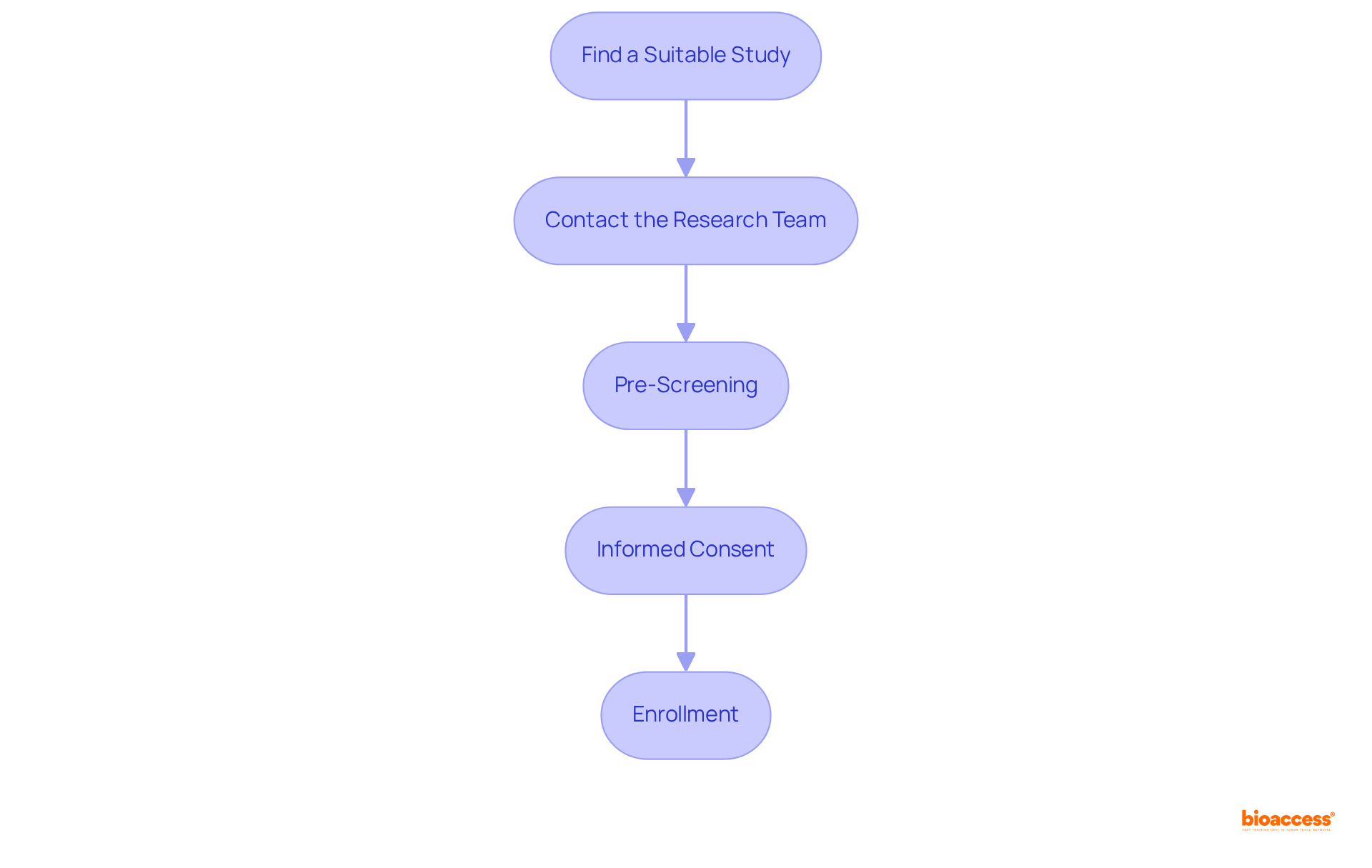
To enroll in a clinical trial, follow these essential steps:
By adhering to these steps, you can effectively enroll in clinical trials that pay, allowing you to contribute to vital medical research while potentially receiving compensation for your participation.
Finding clinical trials that offer compensation for participation presents a valuable opportunity for individuals eager to contribute to medical research while potentially earning financial rewards. By understanding the intricacies of clinical trials—including their purpose, ethical guidelines, and enrollment steps—participants are empowered to make informed decisions regarding their involvement.
This guide outlines key strategies for effectively identifying paid clinical trials. Utilizing reputable registries, understanding eligibility criteria, and evaluating risks and benefits are crucial steps that ensure a safe and rewarding experience. The emphasis on informed consent highlights the necessity of being fully aware of what participation entails.
Engaging in clinical trials not only opens doors to innovative treatments but also plays a vital role in advancing healthcare knowledge. By proactively seeking these opportunities, individuals can contribute to significant medical breakthroughs while receiving compensation for their time and effort. Embrace the chance to be part of the future of medicine—begin exploring clinical trials today.
What are clinical trials?
Clinical trials are structured investigations involving human participants aimed at evaluating the safety and efficacy of new healthcare interventions, such as drugs, devices, and treatment protocols.
Why are clinical trials important?
Clinical trials are crucial for generating information that can lead to groundbreaking treatments or enhance existing therapies, thereby advancing health knowledge and improving healthcare outcomes.
How many clinical studies are currently registered worldwide?
As of 2025, there are over 452,000 registered studies worldwide, with 64,838 actively seeking participants.
What ensures the safety of participants in clinical trials?
Clinical trials are conducted under rigorous ethical and safety guidelines, which are designed to protect participants while striving to improve healthcare outcomes.
What is the historical significance of successful clinical studies?
Successful clinical studies have historically led to innovative treatments, highlighting their importance in the healthcare landscape and the advancement of medical research.
What role do clinical trials play in the validation of new therapies?
The stringent procedures followed in clinical trials are vital for validating new therapies before they become widely available, establishing these investigations as a cornerstone of modern medical research.