

Navigating the biopharma regulatory submission process in North Macedonia presents significant challenges, particularly due to the complexities of the governing framework and the ever-evolving regulatory landscape. This guide aims to clarify the submission process, outlining essential steps that can streamline applications and improve the likelihood of success. Given the potential pitfalls of incomplete documentation, shifting regulations, and communication barriers, stakeholders must ask themselves: how can they adequately prepare to tackle these challenges and secure timely approvals?
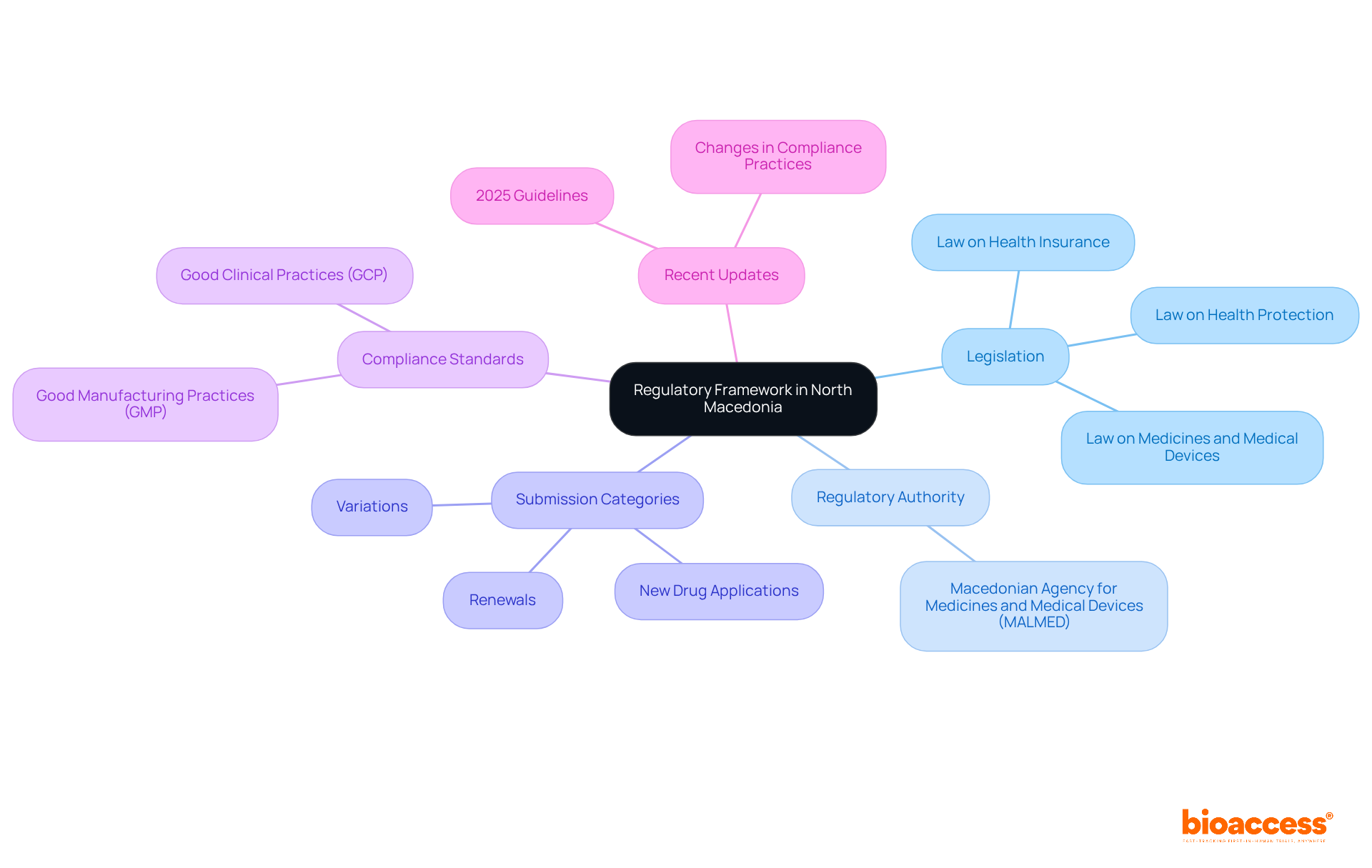
Successfully managing the biopharma regulatory submission process in North Macedonia requires a solid understanding of the governing framework. Familiarizing yourself with the following key components is essential:
Legislation: The primary laws governing biopharmaceuticals in North Macedonia include the Law on Medicines and Medical Devices. This law outlines the requirements for marketing authorization, clinical trials, and product registration. Regulatory expert Aleksandar Josimovski emphasizes, "The Law on Medicines and Medical Devices comprehensively regulates the manner of advertising medicines and medical devices."
Regulatory Authority: The Macedonian Agency for Medicines and Medical Devices (MALMED) oversees the approval process. Understanding the guidelines and procedures of the biopharma regulatory submission process in North Macedonia is crucial for success in proposals. Partnering with bioaccess can provide valuable insights and support, helping you navigate these regulations effectively and ensuring timely regulatory approval.
Submission Categories: Various types of entries exist, including new drug applications, variations, and renewals. Each type has specific requirements and timelines that must be adhered to. Bioaccess can assist in optimizing these entries within the biopharma regulatory submission process in North Macedonia, ensuring that your applications are prepared accurately and efficiently, ultimately speeding up your clinical trials by 40%.
Compliance Standards: Familiarize yourself with the compliance standards established by the organization, such as Good Manufacturing Practices (GMP) and Good Clinical Practices (GCP). Adhering to these standards is vital for ensuring product quality in the biopharma regulatory submission process in North Macedonia and avoiding significant penalties. Bioaccess's expertise in these areas can help mitigate risks associated with compliance.
Recent Updates: Staying informed about changes in legislation or compliance practices is crucial, particularly the 2025 guidelines from the relevant authority, as these can impact the biopharma regulatory submission process in North Macedonia and its timelines. Regularly reviewing resources like the official MALMED website or industry publications will keep you updated on the latest insights into changing compliance environments. Utilizing bioaccess's services can significantly enhance your chances of obtaining successful approval.
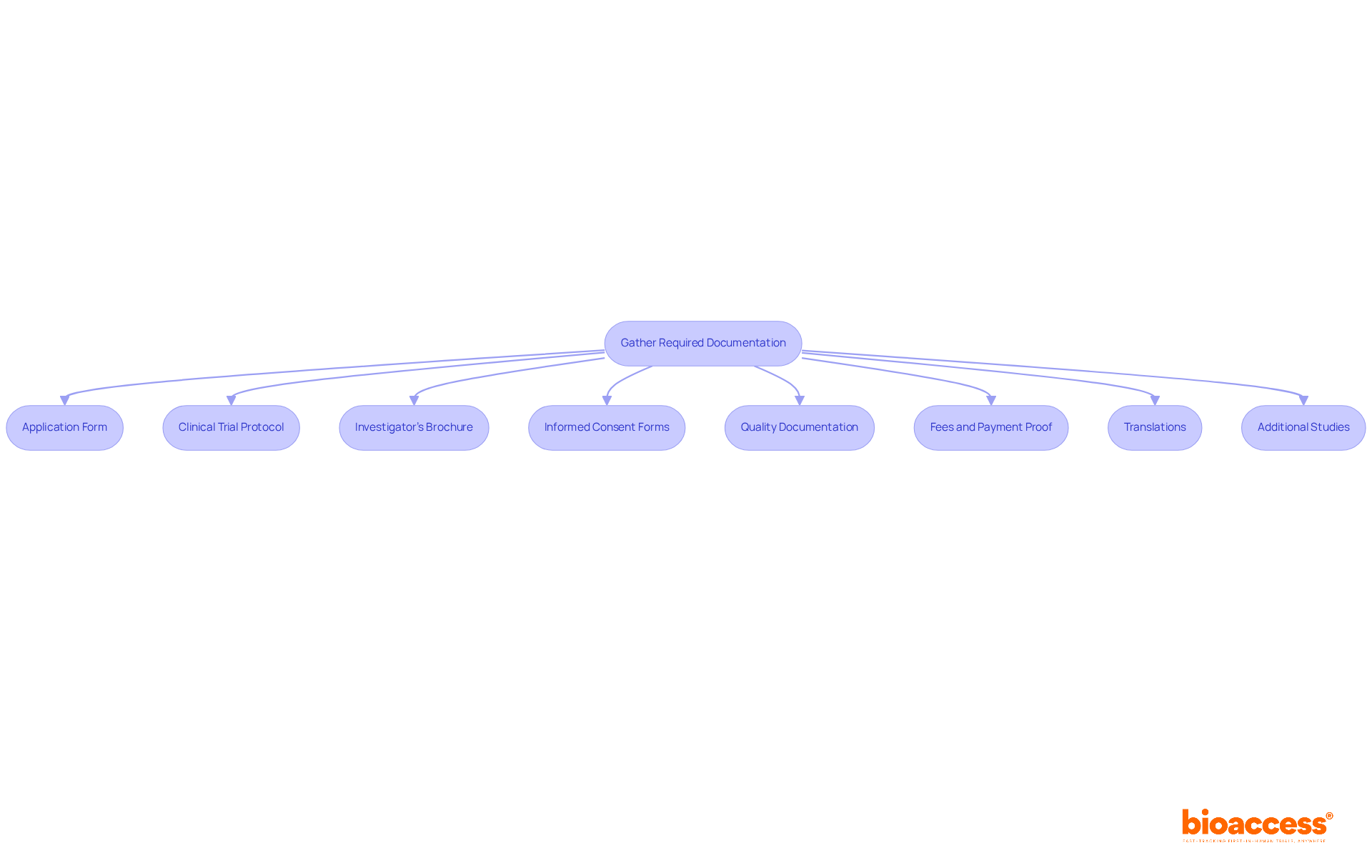
To successfully navigate the biopharma regulatory submission process in North Macedonia, it is crucial to systematically gather all necessary documentation and submission materials. This typically includes:
Arranging these documents methodically will enable a smoother filing experience, increasing the chances of compliance approval. Furthermore, performing final editorial reviews to guarantee data consistency before submission is essential. Respond swiftly to any inquiries from the oversight agency during the assessment process, and remain updated on AI-driven trends that are transforming life sciences compliance operations. By adhering to these best practices and leveraging Bioaccess's comprehensive clinical trial management services, you can more effectively navigate the biopharma regulatory submission process in North Macedonia.
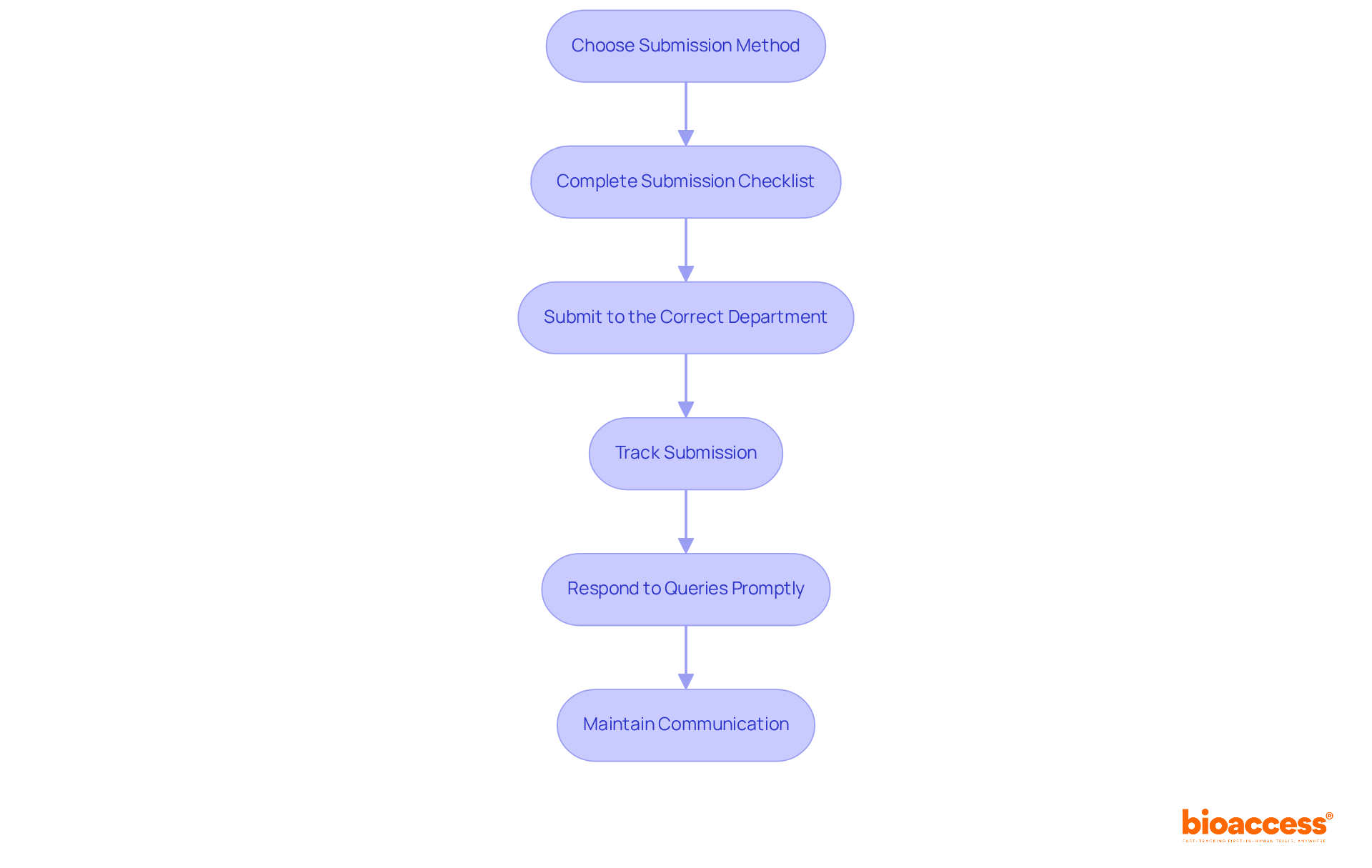
Once all documentation is prepared, the next step is to submit your application. Adhering to established protocols is crucial for a successful submission:
Navigating the biopharma regulatory submission process in north macedonia is crucial in clinical research, yet it often presents various challenges. Understanding these challenges and implementing effective strategies can significantly enhance your submission success rate. Here are some common issues and actionable solutions:
Incomplete Documentation: A robust peer review system is essential. Ensure that all required documents are complete and accurate before delivery. This proactive approach not only reduces the risk of delays caused by missing information but also aligns with bioaccess's commitment to compliance reviews.
Regulatory Changes: Staying informed about changes in regulations or submission requirements is vital. Subscribe to industry newsletters and monitor announcements from relevant authorities. Engaging with regulatory affairs specialists can provide valuable insights into adapting to these changes effectively, a service that bioaccess offers.
Delays in Approval: Anticipate potential delays by constructing a detailed timeline that includes buffer periods for unforeseen issues. Consistent communication with MALMED can clarify typical approval timelines and help identify potential bottlenecks early on. Utilizing bioaccess's project management services can further streamline this process.
Communication Barriers: Language challenges can hinder effective communication. Consider hiring a local compliance consultant whose expertise can facilitate clear communication with MALMED, ensuring compliance with local requirements. Bioaccess can assist in this area by providing experienced professionals.
Resource Limitations: If your team lacks specific expertise, outsourcing tasks to experienced compliance advisors or firms specializing in biopharma applications can be a strategic move. This allows your team to focus on essential activities while ensuring compliance with all regulatory standards, leveraging bioaccess's comprehensive clinical trial management services.
Ethical Concerns: Addressing ethical considerations is paramount. Ensure that all studies comply with established ethical guidelines, and acquiring informed consent from all participants is essential for preserving integrity and trust during evaluations. Bioaccess emphasizes adherence to ethical standards in all its trial setups.
By anticipating these challenges and preparing effective solutions, you can significantly enhance the likelihood of a successful biopharma regulatory submission process in north macedonia and expedite the overall approval process. Collaboration with experts like bioaccess can make a substantial difference in navigating the complexities of regulatory filings.

Successfully navigating the biopharma regulatory submission process in North Macedonia is a complex endeavor that demands a thorough understanding of the regulatory landscape, meticulous documentation preparation, and strategic communication with regulatory bodies. Mastering these elements is crucial for ensuring that biopharmaceutical products meet the necessary approval requirements and can efficiently reach the market.
Key points highlighted in this article underscore the importance of:
Furthermore, the discussion emphasizes the need to anticipate challenges such as:
While also offering actionable solutions to navigate these hurdles. Collaborating with experts like Bioaccess can significantly enhance the submission process, ensuring compliance and expediting approval timelines.
Ultimately, successful navigation of the biopharma regulatory submission process in North Macedonia transcends mere adherence to regulations; it embodies a proactive approach that fosters collaboration, continuous learning, and strategic planning. By staying informed and leveraging specialized support, stakeholders can not only improve their chances of regulatory success but also contribute to advancing healthcare innovations in the region. Taking these steps will ensure that biopharmaceutical advancements are delivered to patients in a timely and efficient manner.
What is the primary law governing biopharmaceuticals in North Macedonia?
The primary law is the Law on Medicines and Medical Devices, which outlines the requirements for marketing authorization, clinical trials, and product registration.
Which authority oversees the approval process for biopharmaceuticals in North Macedonia?
The Macedonian Agency for Medicines and Medical Devices (MALMED) oversees the approval process.
What types of submissions are involved in the biopharma regulatory process?
The submission categories include new drug applications, variations, and renewals, each with specific requirements and timelines.
How can Bioaccess assist in the regulatory submission process?
Bioaccess can provide valuable insights, support in navigating regulations, and help optimize entries, ultimately speeding up clinical trials by 40%.
What compliance standards should be familiarized with in North Macedonia?
Key compliance standards include Good Manufacturing Practices (GMP) and Good Clinical Practices (GCP), which are vital for ensuring product quality and avoiding penalties.
Why is it important to stay updated on regulatory changes in North Macedonia?
Staying informed about changes in legislation or compliance practices, such as the 2025 guidelines, is crucial as they can impact the biopharma regulatory submission process and its timelines.
How can one keep informed about the latest regulatory updates?
Regularly reviewing resources like the official MALMED website or industry publications can help keep you updated on the latest insights into changing compliance environments.