


Navigating the complexities of the early phase trial approval process under NAMMD presents a significant challenge for clinical researchers in Romania. A comprehensive understanding of the regulatory landscape, including key laws and the impact of European Union regulations, is essential for achieving successful outcomes. Many researchers encounter obstacles in preparing the necessary documentation, engaging with regulatory authorities, and managing approval timelines. So, how can researchers effectively navigate this intricate system to ensure their clinical studies not only comply but also thrive?
The Medtech landscape is evolving, and understanding its nuances is crucial for researchers aiming to succeed. Bioaccess plays a pivotal role in addressing the key challenges faced during the approval process. By leveraging expertise and resources, researchers can streamline their approach, ensuring compliance while enhancing the likelihood of successful outcomes. Collaboration and strategic planning are vital components in this journey, enabling researchers to overcome hurdles and achieve their goals.
Navigating the early phase trial approval process under NAMMD is crucial for anyone involved in clinical research in Romania. A strong understanding of the regulatory framework is essential, particularly Law 95/2006, which outlines healthcare reform and establishes the requirements for clinical trial applications. This legislation, along with its modifications, significantly shapes the endorsement process and ensures adherence to ethical standards.
Moreover, the influence of European Union regulations on Romanian law is substantial, as the authority operates within this broader context. For those seeking comprehensive insights into the regulatory environment, including authorization timelines, necessary documentation, and compliance expectations, resources such as the relevant website and official publications are indispensable. Understanding these components is vital for achieving successful clinical studies in Romania.
As you consider your own challenges in clinical research, reflect on how these regulations impact your work. Are you fully aware of the requirements and resources available to navigate this complex landscape? By understanding the intricacies of the early phase trial approval process under NAMMD, you position yourself for success in the Medtech field.

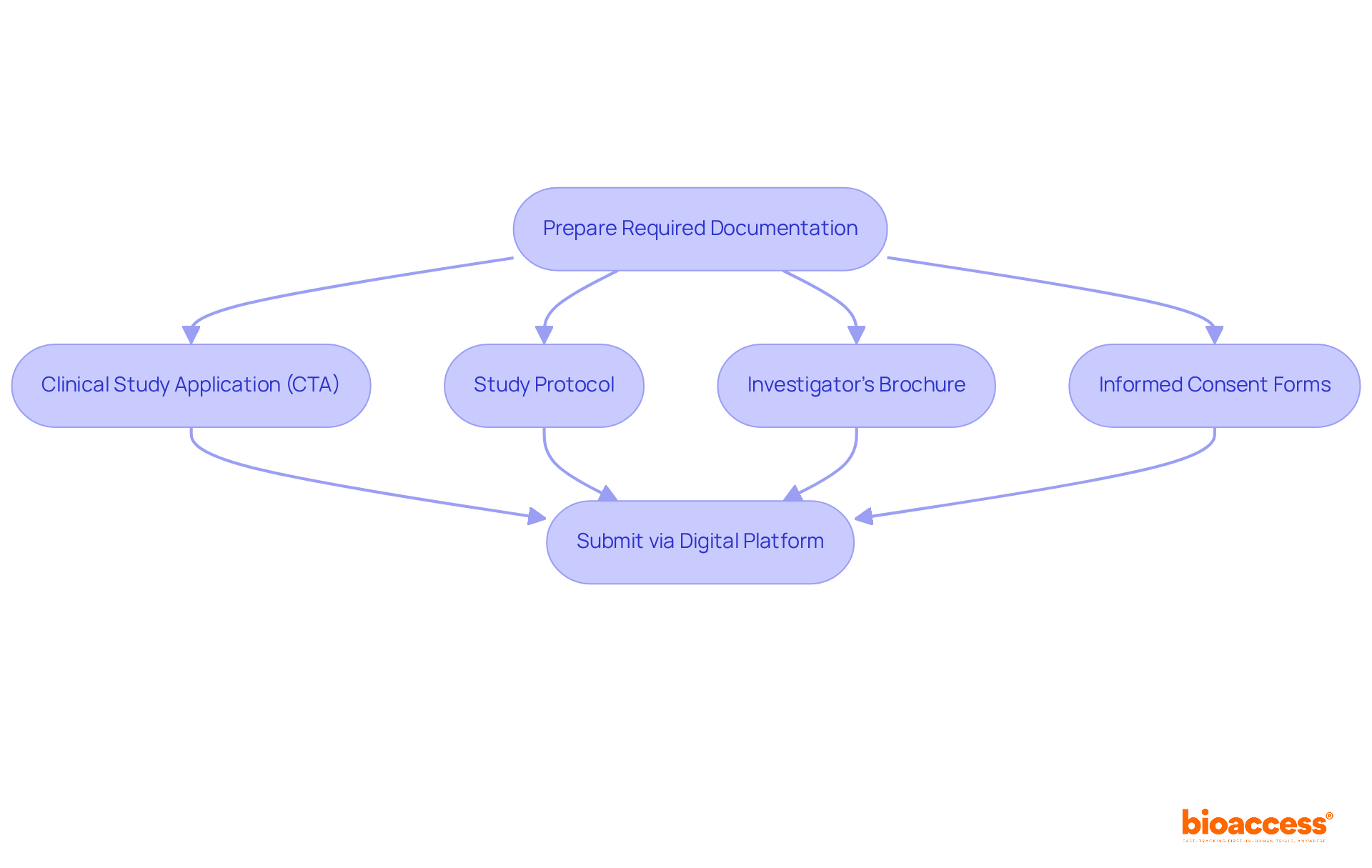
Preparing the necessary paperwork is crucial in the early phase trial approval process under NAMMD within the relevant regulatory framework. Key documents such as the clinical study application (CTA), study protocol, investigator's brochure, and informed consent forms must be meticulously crafted to comply with the early phase trial approval process under NAMMD.
Begin by developing a comprehensive study protocol that clearly outlines the study's objectives, methodology, and statistical analysis plan. The investigator's brochure should include all relevant information about the investigational product, encompassing safety data and dosing guidelines. Informed consent forms need to be clear and informative, ensuring that potential participants fully understand the trial's nature and their rights.
Once these documents are compiled, submit them through the organization's digital platform, making sure all files adhere to the required format and language specifications. Regularly check the relevant website for updates on submission guidelines to ensure compliance with any regulatory changes. This diligence not only streamlines the approval process but also enhances the integrity of the clinical research endeavor.
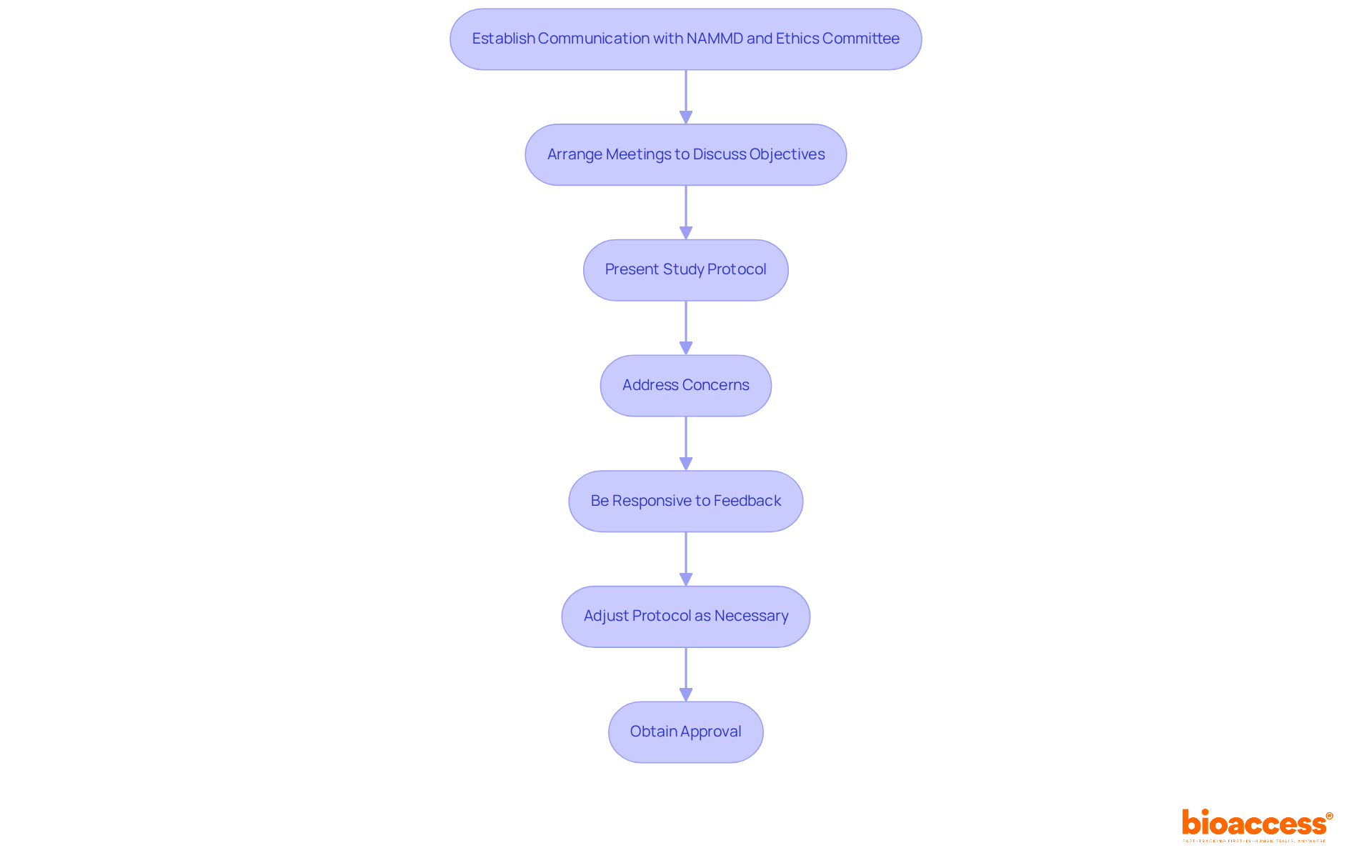
Engaging effectively with regulatory authorities and ethics committees is essential for the success of your clinical study. Establishing clear communication channels with the National Agency for Medicines and Medical Devices (NAMMD) and the relevant ethics committee is crucial for the early phase trial approval process under NAMMD. Arrange meetings to discuss your test's objectives and address any concerns they may have. Present your study protocol and documentation concisely, emphasizing ethical considerations and the potential benefits of the trial.
Being responsive to feedback and prepared to adjust your protocol as necessary fosters clarity during the procedure, promoting confidence and potentially accelerating endorsements. Utilize resources like official guidelines and examples of past successful applications to enhance your submissions. This proactive strategy simplifies the authorization workflow and aligns with the evolving regulatory environment in Romania, where the early phase trial approval process under NAMMD is increasingly recognized as a vital element in facilitating clinical research.
Navigating the endorsement schedules and expectations set by the organization is crucial for effective project management in clinical studies. The approval process typically spans up to 60 days from the submission of a complete application, but this duration can vary based on trial complexity and the thoroughness of the documentation provided. To minimize potential delays, it’s prudent to allocate extra time for possible revisions or requests for additional information from the relevant organization.
Consistently checking the status of your application through the digital platform is essential, as is being prepared to respond promptly to any inquiries. Establishing internal deadlines that align with the early phase trial approval process under NAMMD can help maintain project momentum and ensure that all stakeholders are kept informed about progress. This proactive approach not only enhances compliance but also cultivates a culture of continuous improvement, ultimately leading to better patient outcomes and more successful trials.

Navigating the early phase trial approval process under NAMMD is crucial for anyone involved in clinical research in Romania. A solid understanding of the regulatory framework, especially Law 95/2006 and its implications, is vital for successful clinical studies. This knowledge not only ensures compliance with ethical standards but also significantly increases the chances of obtaining timely approvals.
To streamline this process, there are four key steps to consider:
Each of these steps is essential for conducting clinical trials efficiently and ethically, ultimately driving the advancement of medical research.
In summary, being well-prepared and proactive is paramount. By familiarizing yourself with NAMMD regulations, meticulously preparing documentation, maintaining open communication with authorities, and effectively managing timelines, researchers can greatly enhance their chances of success. Embracing these best practices not only benefits individual projects but also fortifies the overall landscape of clinical research in Romania, paving the way for groundbreaking medical advancements.
What is the NAMMD regulatory framework?
The NAMMD regulatory framework governs the early phase trial approval process for clinical research in Romania, ensuring compliance with ethical standards and legal requirements.
What legislation is central to the NAMMD framework?
Law 95/2006 is central to the NAMMD framework, as it outlines healthcare reform and establishes the requirements for clinical trial applications.
How do European Union regulations influence Romanian clinical trial laws?
European Union regulations significantly influence Romanian law, as the NAMMD operates within this broader regulatory context.
What resources are recommended for understanding the regulatory environment in Romania?
Comprehensive insights can be gained from the relevant website and official publications that detail authorization timelines, necessary documentation, and compliance expectations.
Why is it important to understand the NAMMD regulatory framework for clinical studies in Romania?
Understanding the NAMMD regulatory framework is vital for achieving successful clinical studies, as it helps researchers navigate the complex approval process and adhere to necessary standards.
What should researchers reflect on regarding the NAMMD regulations?
Researchers should consider how the NAMMD regulations impact their work and whether they are fully aware of the requirements and resources available to navigate the regulatory landscape.