


The article delineates four pivotal strategies for successful paid recruitment in clinical studies. It emphasizes the importance of:
These strategies are underpinned by compelling evidence indicating that targeted recruitment can significantly elevate enrollment rates. Furthermore, effective data management and compliance are essential for upholding the integrity and efficiency of clinical trials. This comprehensive approach not only addresses key challenges in the Medtech landscape but also highlights the necessity of collaboration in advancing clinical research.
Navigating the landscape of clinical studies demands a profound understanding of various phases and methodologies, especially as the need for innovative medical solutions escalates. Successful recruitment strategies are crucial for ensuring these studies not only comply with regulatory standards but also attract the appropriate participants. Yet, as competition intensifies and the recruitment process becomes increasingly complex, how can researchers effectively engage potential volunteers and streamline enrollment? This article delves into four essential strategies for enhancing paid recruitment in clinical studies, offering insights that can significantly elevate participant engagement and improve study outcomes.
Clinical trials are meticulously structured investigations aimed at evaluating the safety and efficacy of medical interventions. These trials progress through several distinct phases:
Grasping these phases is vital for researchers, as it allows them to create investigations that adhere to regulatory standards and effectively tackle specific research inquiries.
Acquaintance with different research designs, such as randomized controlled trials (RCTs) and observational analyses, is crucial for choosing the suitable methodology adapted to the research question at hand. RCTs, in particular, are regarded as the gold standard in medical research due to their capacity to reduce bias and determine causality. This essential knowledge enables researchers to interact effectively with stakeholders while also guaranteeing that investigations are carried out systematically and ethically. Importantly, a considerable percentage of trials fail due to a lack of understanding of these essential research designs, highlighting the necessity of thorough training and education in research methodologies.
Additionally, bioaccess® has more than 20 years of expertise in Medtech, providing expedited medical device research services in Latin America. With a focus on specialized knowledge and flexibility, bioaccess® manages Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies efficiently. Ethical approvals are delivered in just 4-6 weeks, facilitating timely patient enrollment and ensuring significant cost savings of $25K per patient. Collaboration with bioaccess® not only addresses key challenges in clinical research but also enhances the overall efficiency and effectiveness of clinical studies paid.
Compliance with regulatory standards, such as those set by the FDA or EMA, is essential for the successful conduct of clinical studies. At bioaccess, we facilitate the feasibility and selection of research sites and principal investigators (PIs), ensuring that all protocols are thoroughly reviewed and comply with country requirements. Researchers must ensure that all protocols are approved by an Institutional Review Board (IRB) and that informed consent is acquired from individuals.
Our services encompass trial setup, start-up, and obtaining necessary approvals from ethics committees and health ministries, which are crucial for regulatory compliance. Ethical considerations, including the risk-benefit ratio and confidentiality of subjects, must be prioritized.
Regular training on Good Clinical Practice (GCP) guidelines can help teams stay updated on compliance requirements. By fostering a culture of ethical research, organizations can enhance safety for subjects and data integrity, ultimately resulting in more reliable research outcomes.
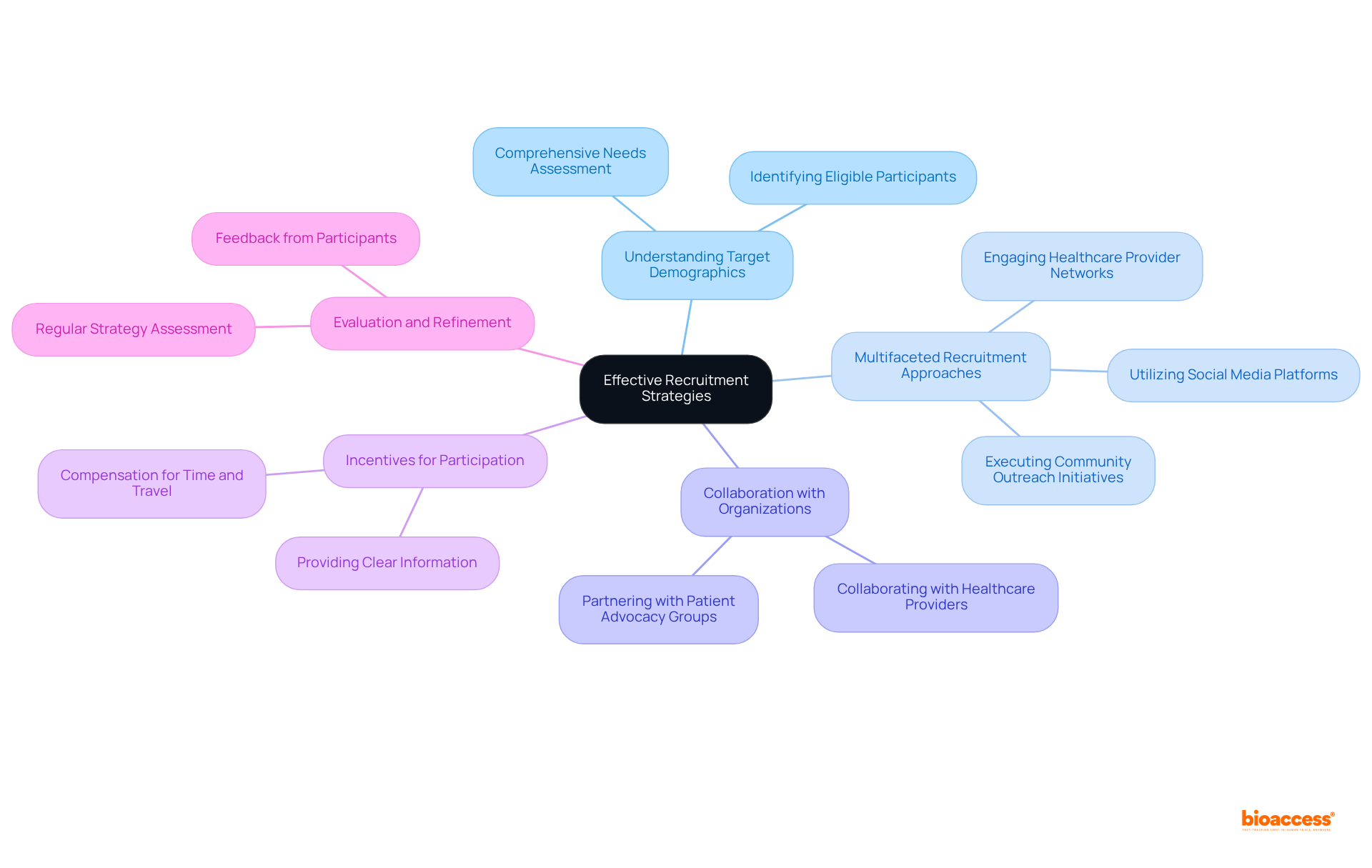
Enlisting individuals for research studies presents significant challenges, yet implementing targeted strategies can markedly improve enrollment numbers. A crucial initial step involves a comprehensive understanding of the demographics and specific needs of your target population. For example, bioaccess® achieves enrollment rates that are 50% faster than traditional markets, illustrating the power of strategic recruitment. This effectiveness is further reinforced by the collaboration between bioaccess™ and Caribbean Health Group, which aspires to establish Barranquilla as a premier destination for clinical trials in Latin America, with the backing of Colombia's Minister of Health.
Adopting a multifaceted recruitment approach is essential; this encompasses:
Collaborating with patient advocacy organizations not only raises awareness but also fosters trust among potential volunteers, thereby enhancing recruitment efforts. Moreover, providing incentives, such as compensation for time and travel, can effectively encourage individuals to participate in clinical studies paid.
Regularly evaluating recruitment strategies and refining them based on feedback from participants and enrollment data is vital for improving efforts throughout the research process. By concentrating on these strategies, organizations can adeptly navigate the complexities of participant recruitment, ultimately contributing to job creation and economic growth in the region.
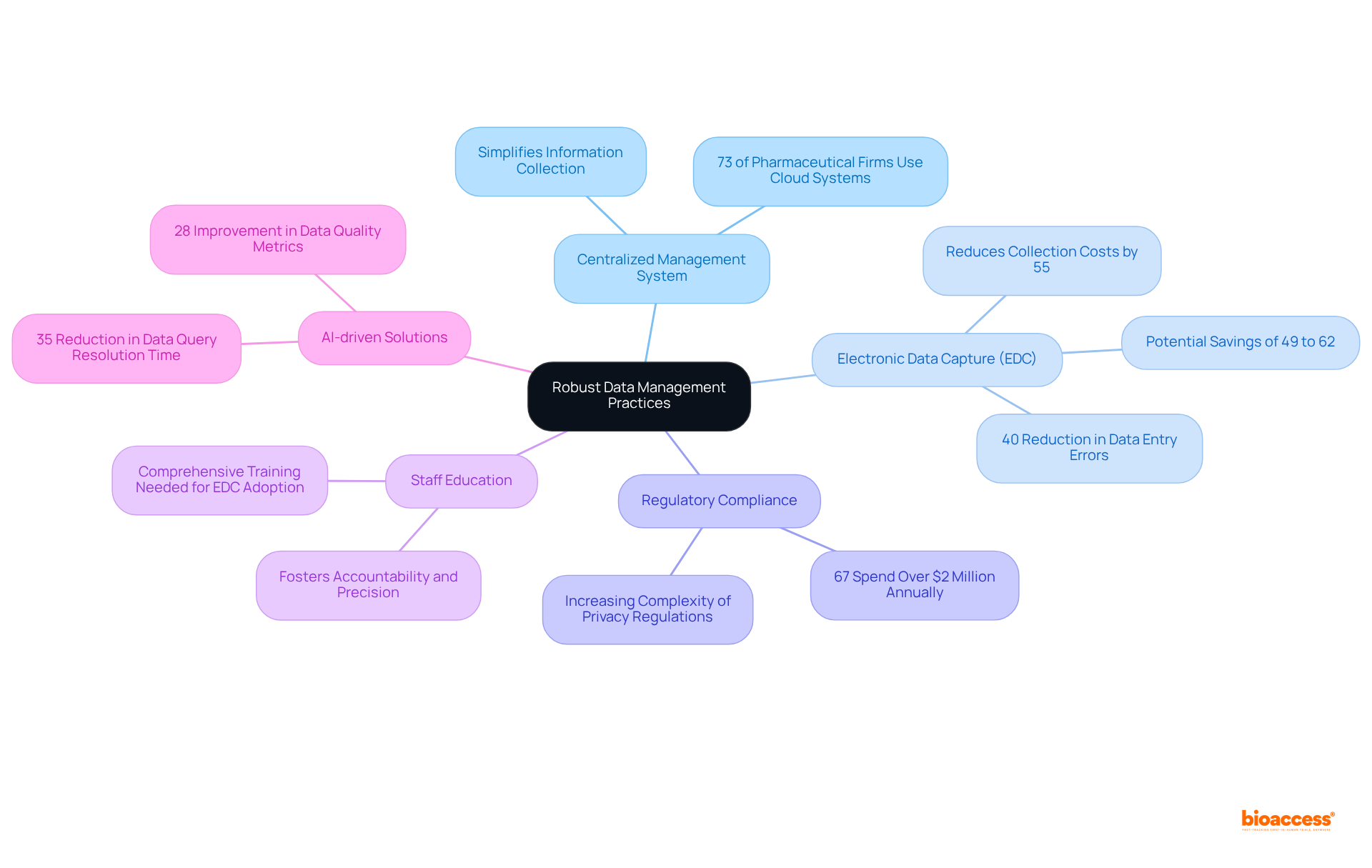
Efficient information management is paramount for the success of research studies. Establishing a centralized information management system simplifies the collection of information and ensures uniformity across various locations. Currently, over 73% of pharmaceutical firms utilize cloud-based clinical information management systems, demonstrating a significant shift towards agile solutions.
The adoption of electronic data capture (EDC) systems mitigates errors and enhances accuracy, with research indicating that EDC can reduce collection costs by as much as 55%, yielding potential savings between 49% and 62% compared to traditional paper-based methods. Regular audits and validation checks are essential for early identification of discrepancies, thereby maintaining integrity throughout the research process.
Furthermore, secure information storage in compliance with regulatory standards is critical, especially given the increasing complexity of privacy regulations, which 67% of pharmaceutical firms report managing at a cost exceeding $2 million annually. Educating staff on information management protocols fosters a culture of accountability and precision, ultimately leading to more reliable research outcomes.
Optimal strategies for information management in research studies necessitate proactive engagement with personnel to address challenges in EDC adoption, such as resistance to change and the need for comprehensive training. Case studies reveal that successful implementation can result in significant improvements in accuracy and operational efficiency.
As Sarah Lee observed, clinical trials that incorporate AI-driven data management solutions experience a 35% reduction in data query resolution time and a 28% enhancement in data quality metrics.
Understanding the intricacies of clinical studies is crucial for researchers aiming to conduct effective and ethical medical investigations. By grasping the fundamental phases of clinical trials—from safety assessments in Phase I to efficacy comparisons in Phase III—researchers can ensure their studies are well-structured and compliant with regulatory standards. This foundational knowledge facilitates smoother interactions with stakeholders and enhances the overall integrity of the research process.
The article highlights several key strategies for successful participant recruitment, emphasizing the importance of tailored approaches that resonate with target demographics. Utilizing social media, engaging healthcare networks, and fostering community outreach are essential tactics that can significantly improve enrollment rates. Moreover, maintaining compliance with ethical standards and regulatory guidelines ensures that studies are conducted responsibly, safeguarding participant welfare while enhancing the credibility of the research outcomes.
Ultimately, the success of clinical studies hinges on a combination of robust recruitment strategies, adherence to ethical practices, and effective data management. By prioritizing these elements, researchers can navigate the complexities of clinical trials more effectively, paving the way for innovations that advance healthcare. Organizations are encouraged to adopt these best practices and continually refine their approaches, ensuring that clinical research not only meets regulatory expectations but also contributes positively to community health and well-being.
What are clinical trials?
Clinical trials are structured investigations aimed at evaluating the safety and efficacy of medical interventions.
What are the different phases of clinical trials?
Clinical trials progress through several phases: Phase I focuses on safety and dosage, involving 20 to 100 healthy volunteers. Phase II assesses efficacy with 100 to 300 participants. Phase III compares the new intervention against standard treatments, often involving 300 to 3,000 participants over 1 to 4 years.
Why is understanding the phases of clinical trials important for researchers?
Understanding the phases is vital for researchers to create investigations that adhere to regulatory standards and effectively address specific research inquiries.
What types of research designs are important in clinical studies?
Important research designs include randomized controlled trials (RCTs) and observational analyses. RCTs are considered the gold standard in medical research due to their ability to reduce bias and determine causality.
What challenges can arise from a lack of understanding of research designs?
A significant percentage of trials fail due to a lack of understanding of essential research designs, which underscores the necessity for thorough training and education in research methodologies.
What services does bioaccess® provide in the field of clinical research?
Bioaccess® offers expedited medical device research services in Latin America, managing Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies efficiently.
How quickly can ethical approvals be obtained through bioaccess®?
Ethical approvals can be delivered in just 4-6 weeks, facilitating timely patient enrollment.
What cost savings can be achieved by collaborating with bioaccess®?
Collaborating with bioaccess® can ensure significant cost savings of $25K per patient.