


The article titled "7 Benefits of Paid Clinical Studies for Participants" focuses on the significant advantages individuals can gain by participating in paid clinical trials. While the specific content of the article is not provided, it can be inferred that the benefits may encompass:
Various organizations emphasize the critical importance of participant engagement, highlighting the positive impacts of clinical studies on both individual and community health.
The realm of clinical research is undergoing rapid evolution, presenting participants with unparalleled opportunities to contribute to medical advancements while enjoying personal benefits. Engaging in paid clinical studies not only grants access to innovative treatments and financial compensation but also plays a vital role in shaping the future of healthcare.
However, with a plethora of options available, what are the genuine advantages of participating in these studies, and how can individuals effectively navigate the complexities of involvement? This article delves into the multifaceted benefits of joining paid clinical studies, illuminating the transformative impact they can have on both participants and the broader medical community.
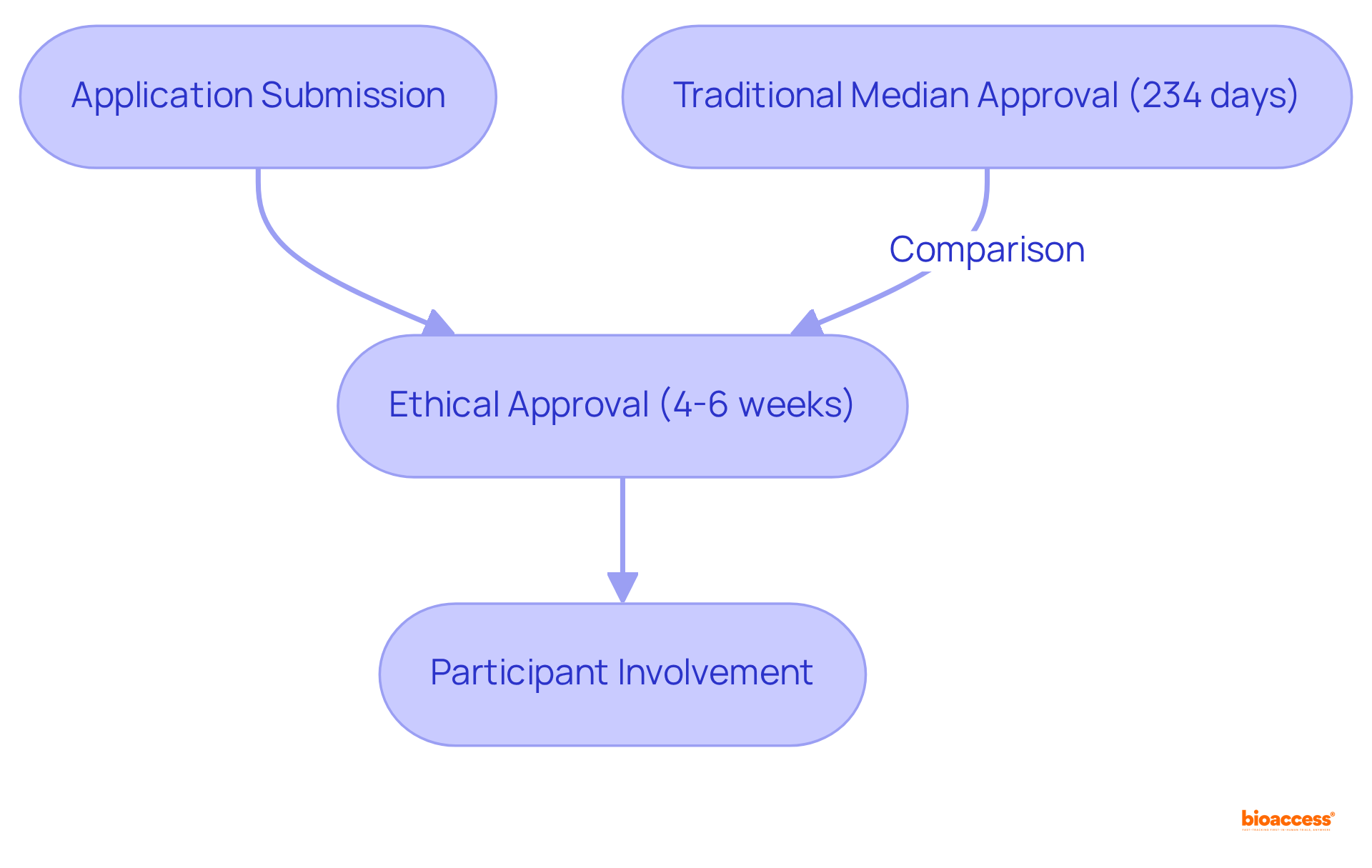
bioaccess® harnesses the regulatory speed of Latin America to secure ethical approvals in just 4 to 6 weeks. This rapid shift significantly reduces waiting periods for individuals, enabling them to participate in paid clinical studies without extended delays. In comparison, the overall median time to site activation for clinical studies is 234 days, underscoring the efficiency of bioaccess®'s process. This swift approval not only boosts involvement in paid clinical studies but also makes it an attractive option for those eager to contribute to medical progress.
With Colombia's healthcare system rated among the best globally and notable cost reductions exceeding 30% compared to North America and Western Europe, individuals can be assured of the quality and affordability of paid clinical studies they engage in.
As Sam Crosby from The George Institute for Global Health observes, 'The results from multi-centre studies are essential to the practice of evidence-based medicine, facilitating the creation and application of new treatments.'
By prioritizing efficiency and leveraging Colombia's robust healthcare infrastructure, bioaccess® guarantees that individuals experience a streamlined process that respects their time and dedication, ultimately enhancing their engagement in paid clinical studies.
As the landscape of clinical research evolves in 2025, the importance of rapid approvals becomes increasingly evident, with organizations like bioaccess® leading the way in fostering participant-centric approaches.
Centricity Research provides individuals with the opportunity to access innovative therapies that are not yet available to the general public. In addition to the potential health benefits, participants in paid clinical studies often receive financial compensation for their time and involvement. This combination of groundbreaking healthcare strategies and financial incentives makes participation in research studies an attractive option for many. Notably, a significant percentage of study volunteers are involved in paid clinical studies, with payments ranging from $75 to $4,500, depending on the study’s requirements and duration.
Healthcare professionals acknowledge the critical role of financial incentives in paid clinical studies, emphasizing their ability to motivate participation while ensuring that individuals are fully informed about the associated risks and benefits. As research studies evolve, organizations like Centricity Research are pivotal in bridging innovative medical advancements with the individuals who stand to benefit from them.
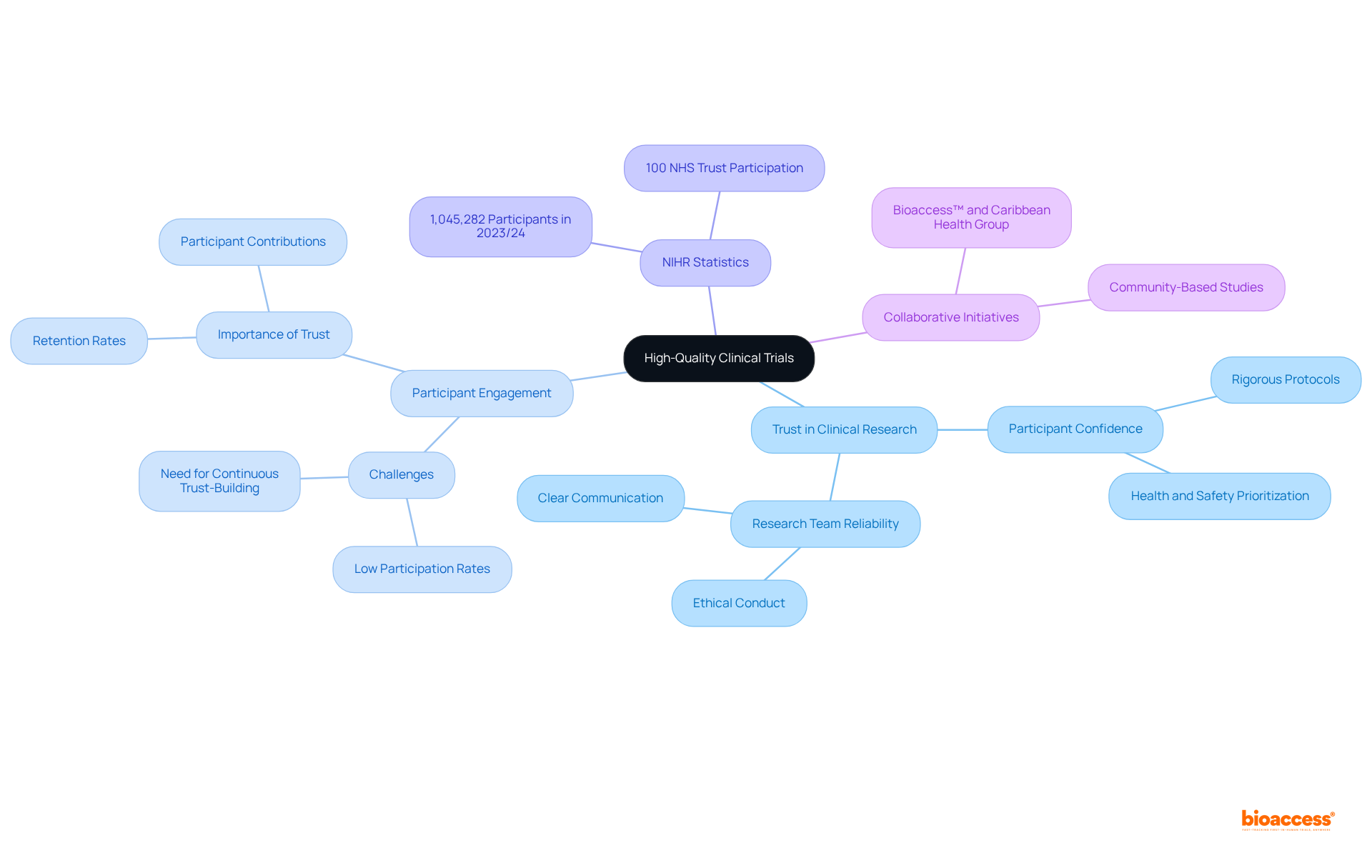
Mayo Clinic distinguishes itself through its unwavering commitment to high-quality clinical research. Participants in their studies reap the benefits of stringent protocols and comprehensive oversight, which are essential for ensuring reliable and beneficial outcomes. This steadfast dedication to maintaining strict standards fosters a robust sense of trust among prospective participants, assuring them that their health and safety are prioritized throughout the study.
In 2023/24, over 1,045,282 individuals in England participated in studies supported by the NIHR Clinical Research Network, reflecting a growing awareness of the importance of trust in medical evaluations. As Ken Getz, a noted expert in medical research, points out, the reliability of study results hinges on the trust established between subjects and research teams. Organizations like Mayo Clinic exemplify this principle, illustrating that rigorous adherence to protocols not only bolsters participant confidence but also propels the advancement of medical knowledge and improved health outcomes.
Furthermore, initiatives such as the collaboration between bioaccess™ and Caribbean Health Group aim to position Barranquilla as a premier research hub in Latin America, with the endorsement of Colombia's Minister of Health. This partnership enhances research management services, encompassing feasibility studies, site selection, compliance assessments, and project oversight, ultimately impacting local economies through job creation and healthcare improvements.
It is crucial to acknowledge that only 5% of eligible patients engage in research studies, highlighting the challenges of attracting individuals and the vital role of trust in overcoming these barriers. Trust is not merely a one-time achievement; it requires continuous effort throughout the process to ensure participant retention and engagement.
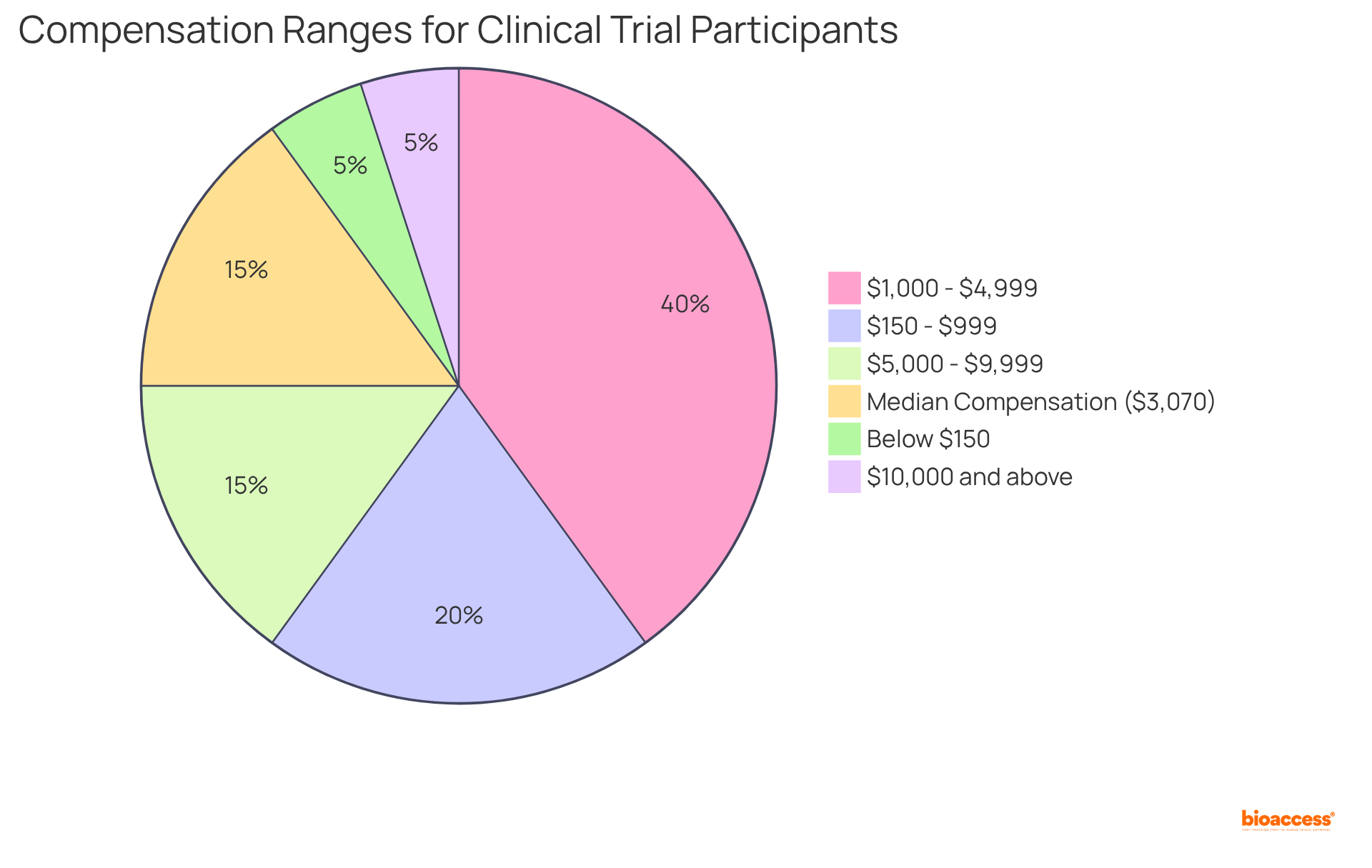
Individuals engaged in clinical studies can expect competitive remuneration that varies significantly based on the study's complexity and duration. Payments typically range from $150 to $13,000, with a median compensation of approximately $3,070. For example, Phase I studies, which often demand more time and involve higher risks, generally provide greater financial rewards compared to later phases. Some rigorous experiments may even exceed $10,000, although such instances are relatively uncommon. This financial aspect is particularly crucial for many individuals, especially those with lower annual incomes, as it can assist in covering essential living expenses like rent and groceries.
Moreover, ethical considerations surrounding compensation are increasingly acknowledged. Clinical study coordinators emphasize the importance of equitable remuneration to ensure that individuals are not unduly influenced by monetary incentives. As one expert observed, while compensation is a factor, it should not serve as the primary motivation for participation. This balance is vital for preserving the validity of medical research while recognizing the financial challenges faced by numerous contributors.
Statistics indicate that a significant portion of participants earn less than $10,000 annually from their involvement, underscoring the need for realistic expectations regarding earnings. Indeed, the highest 10% of earners in research studies reported a median yearly income of $18,885, illustrating the variability in compensation across various investigations. Ultimately, understanding the financial benefits associated with participation in medical studies can empower individuals to make informed decisions regarding their involvement in healthcare initiatives.
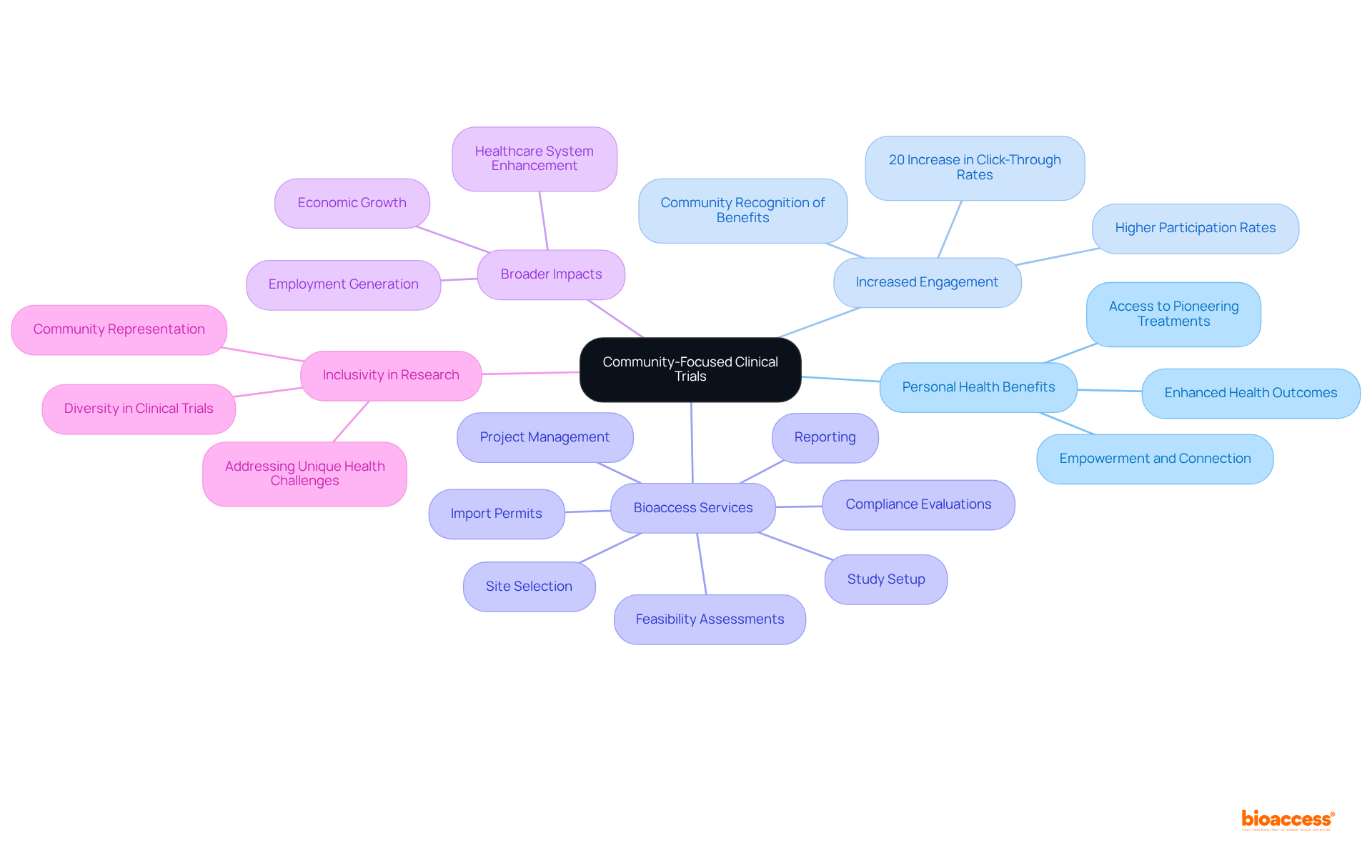
Health Research BC underscores the critical role of community-oriented trials, which not only propel medical advancement but also offer substantial personal health benefits to participants. Engaging in local studies allows individuals to contribute meaningfully to research that directly influences their community's health landscape. Participants frequently gain early access to pioneering treatments and healthcare solutions tailored to their specific needs, cultivating a sense of empowerment and connection within the healthcare system, thereby enhancing overall health outcomes.
Furthermore, local studies have shown a marked increase in participant engagement in medical research, as individuals are more likely to participate when they recognize the direct benefits to their community. For instance, recent data indicates that community-based studies have led to increased awareness and participation rates, with recruitment outreach click-through rates rising by 20% during the pandemic. Researchers assert that local studies not only enrich medical knowledge but also foster a more inclusive environment for diverse populations, ultimately resulting in improved health solutions for all.
As specialists emphasize, integrating local perspectives into medical studies is vital for developing effective therapies that address the unique health challenges of the community. Bioaccess offers comprehensive research study management services, including:
These services ensure that medical studies are conducted efficiently and ethically, contributing to the overall success of research initiatives. Moreover, the impact of Medtech research studies transcends individual health benefits; they generate employment, stimulate economic growth, and enhance healthcare systems in the regions where they are implemented.
As Allison Kalloo, Clinical Ambassador, points out, 'Until individuals of color are a significant part of all medical studies being conducted, best practices for our care will only be a pipe dream.' This statement underscores the necessity of inclusive research practices that mirror the community's diversity.
Eczema Help inspires individuals experiencing eczema to engage in research aimed at developing innovative therapies. By participating, individuals not only contribute to the advancement of medical science but also gain access to potential new therapies that could significantly alleviate their symptoms. This dual impact highlights the importance of participation, as it can lead to improved treatment options that directly benefit those affected by eczema.
Clinical studies have demonstrated their capacity to enhance drug development success rates, with contributions from individuals playing a vital role in refining treatment strategies. Recent research indicates that greater satisfaction with existing therapies correlates with increased interest in research participation, illustrating how participant feedback can shape future treatment environments.
Beginning in 2025, ongoing studies will actively explore new approaches for eczema treatment, showcasing a commitment to enhancing patient outcomes through collaborative research initiatives. Notably, the number of research studies associated with eczema has increased by 3.3 times since 2008, underscoring the expanding opportunities for involvement.
Bioaccess provides comprehensive services for managing paid clinical studies, which include:
By participating in paid clinical studies, individuals not only aid in advancing breakthroughs in eczema treatment but also position themselves at the forefront of new therapeutic options. To learn more about engaging in these studies, consider reaching out to bioaccess for guidance on how to get involved.
CT Ontario provides essential materials to educate individuals about research studies and their benefits. Understanding the purpose, procedures, and potential outcomes of medical studies empowers individuals to make informed decisions regarding their participation. This educational approach fosters transparency and trust, encouraging more individuals to consider engaging in studies.
With bioaccess®, a leading contract research organization, participants can benefit from expedited studies conducted by a team of specialists boasting over 20 years of experience in Medtech. Bioaccess® focuses on:
This commitment to excellence and strategic planning enhances the credibility of research studies, making them a more attractive option for prospective participants.
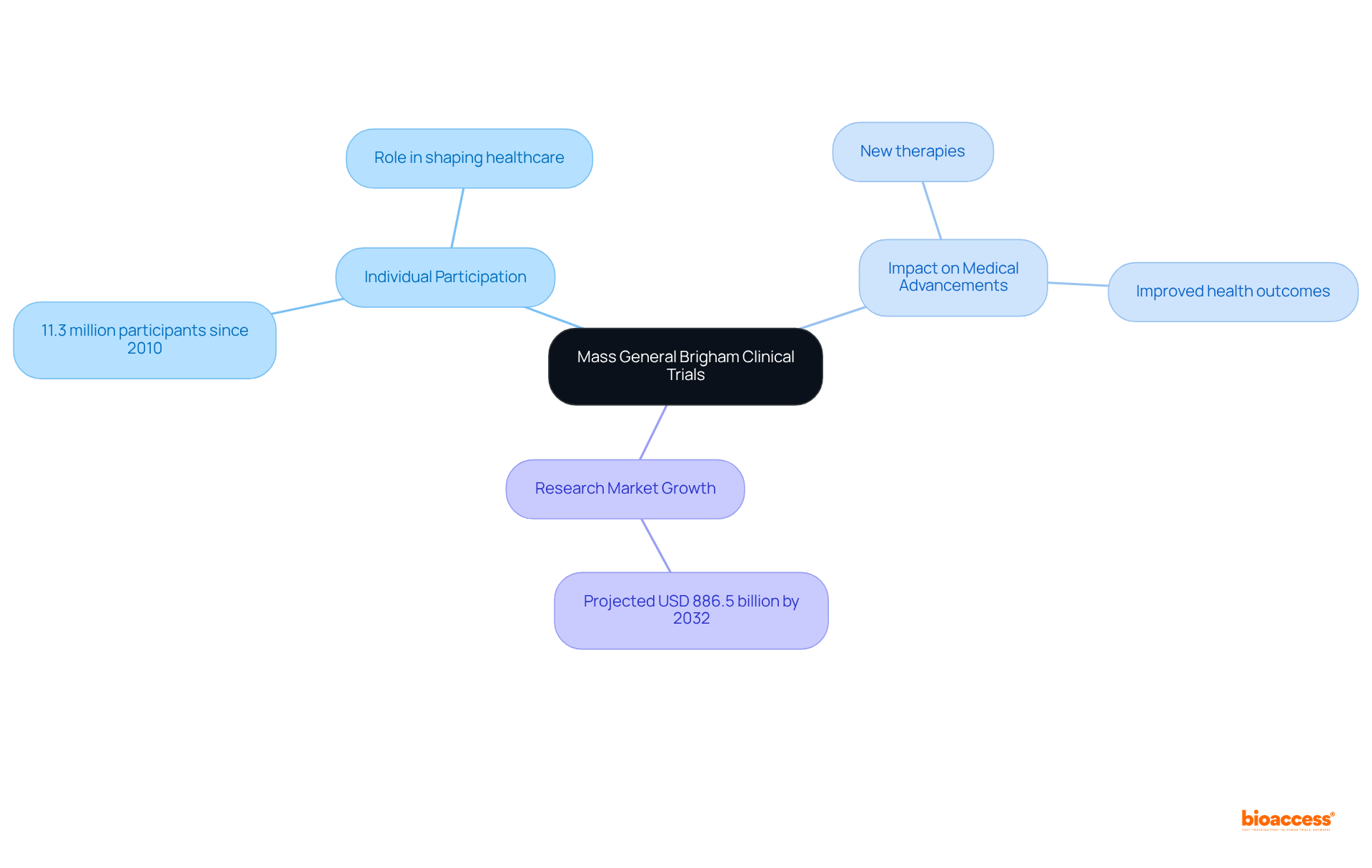
Mass General Brigham calls upon individuals to engage in innovative research studies designed to enhance medical knowledge and treatment options. By participating in these pioneering studies, individuals not only contribute to research that may yield significant breakthroughs across diverse medical fields but also play a crucial role in shaping the future of healthcare.
Notably, statistics reveal that approximately 11.3 million individuals have participated in research studies since 2010, underscoring the collective impact of such involvement on medical advancement. Furthermore, the research market is projected to reach around USD 886.5 billion by 2032, reflecting the growing recognition of the importance of contributor input.
As researchers emphasize, active participation in research studies is vital for developing new therapies and improving health outcomes, making each individual's involvement indispensable in the quest for medical progress.
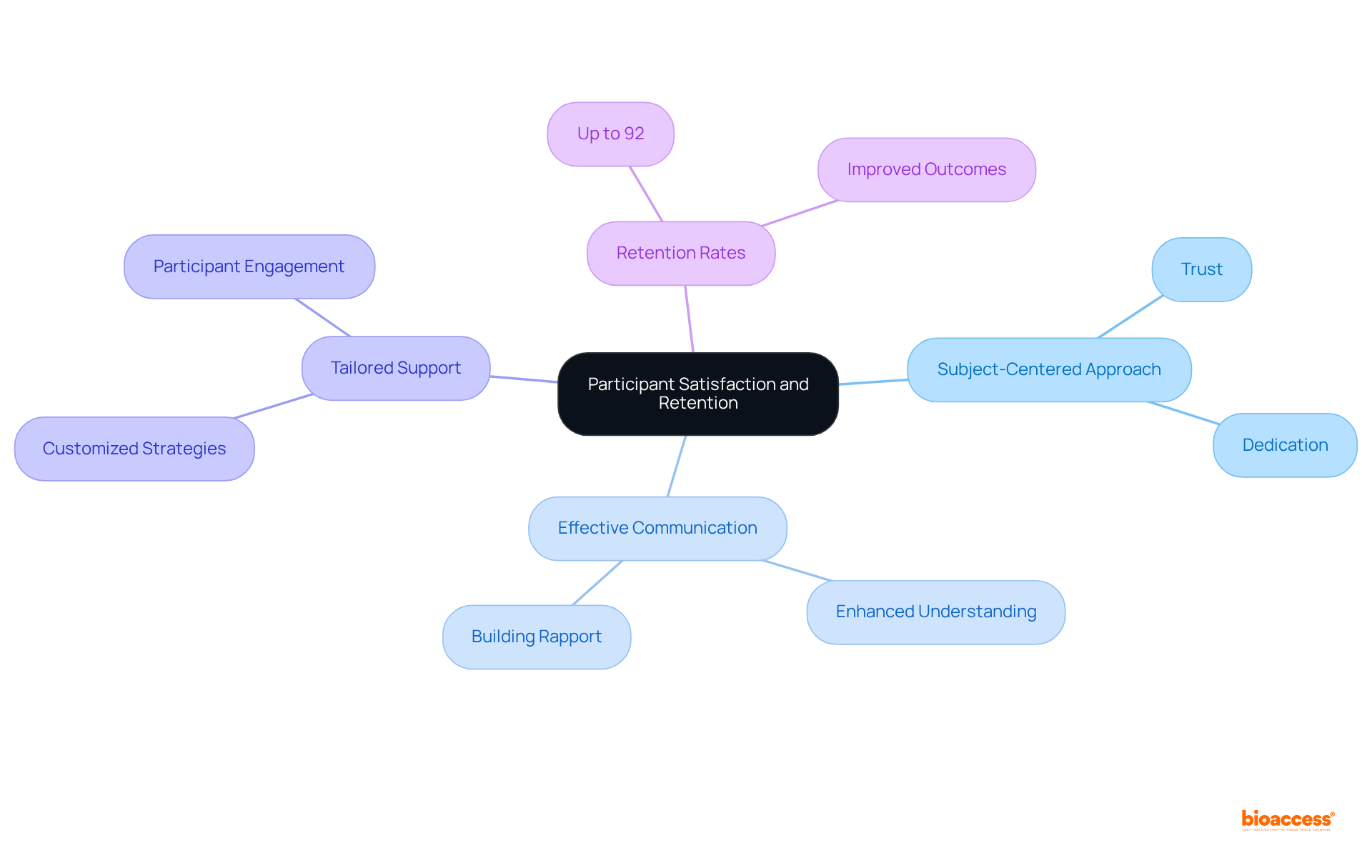
Flourish Research exemplifies a commitment to subject satisfaction and retention within clinical studies, achieving impressive retention rates through a subject-centered approach. By prioritizing the needs and experiences of participants, they create an environment that nurtures trust and dedication. This focus on subject well-being not only enhances the study experience but also significantly contributes to the overall success of the research.
Indeed, studies indicate that effective communication and tailored support can lead to retention rates as high as 92%, underscoring the importance of addressing the concerns and preferences of participants. Organizations that engage actively with participants and implement customized strategies are more likely to witness improved outcomes, highlighting the critical role of participant satisfaction in the success of research studies.
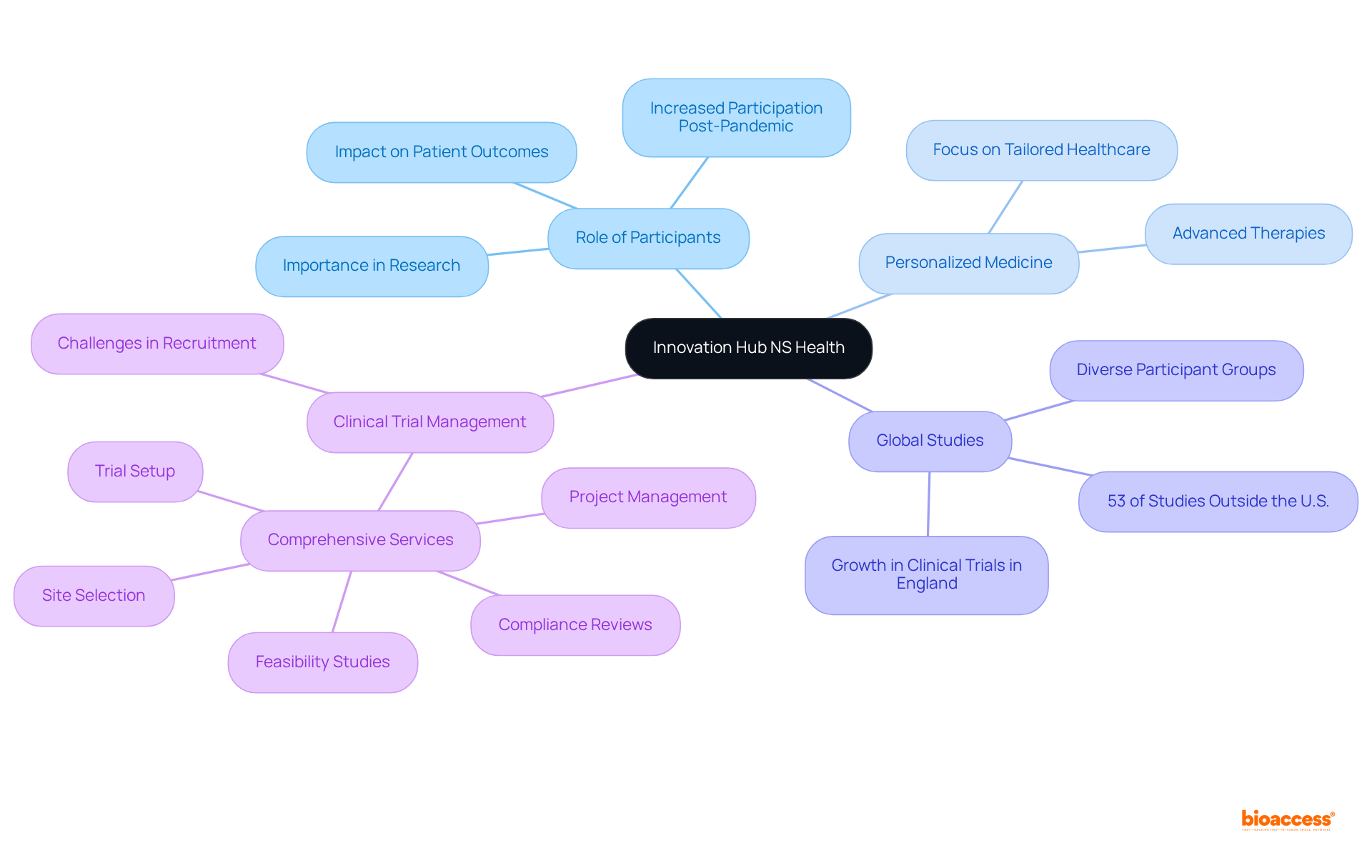
The Innovation Hub NS Health actively encourages individuals to engage in groundbreaking research initiatives that aim to achieve significant health advancements. By participating, individuals not only contribute to studies poised to revolutionize healthcare practices but also play an essential role in enhancing patient outcomes.
In 2025, studies increasingly focus on personalized medicine and advanced therapies, reflecting a pivotal shift towards more tailored healthcare solutions. Participants in these studies are indispensable, as their contributions help shape the future of medical advancements.
Recent data indicates that over 53% of registered studies occur outside the U.S., underscoring the international dimension of medical investigations and the diverse groups involved that bolster the reliability of results. Engaging in these transformative studies provides individuals with compelling reasons to consider enrollment, as they become integral to the journey of healthcare innovation.
As Samruddhi Yardi aptly observes, "Clinical studies are the linchpin of medical progress," emphasizing the vital role participants play in advancing healthcare. The notable increase in research participation in England following the pandemic highlights the growing interest in medical studies and their impact on healthcare practices.
Comprehensive clinical trial management services—encompassing feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting—are crucial for ensuring the success of these initiatives.
Participating in paid clinical studies presents numerous advantages that transcend mere financial compensation. These studies not only provide participants with the opportunity to contribute to groundbreaking medical research but also grant access to innovative treatments and therapies that may not yet be available to the general public. By engaging in clinical trials, individuals play a vital role in advancing healthcare while benefiting personally from potential health improvements associated with new medical interventions.
The article highlights several key benefits of participating in clinical studies, including:
Furthermore, community-focused trials emphasize the importance of local engagement, allowing participants to contribute to research that directly impacts their health and well-being. The growing interest in clinical studies, particularly in the wake of recent global health challenges, underscores the necessity for inclusive and diverse participation to enhance the validity and applicability of research outcomes.
In conclusion, the significance of participating in clinical studies cannot be overstated. Individuals not only stand to gain from financial and health benefits but also have the opportunity to make meaningful contributions to medical science. As the landscape of clinical research continues to evolve, embracing participation in these studies is crucial for fostering innovation and improving healthcare outcomes for all. Engaging in clinical trials is not merely a personal decision; it represents a collective step toward advancing medical knowledge and achieving better health solutions for communities worldwide.
What is bioaccess and how does it benefit participants in clinical studies?
bioaccess® accelerates participation in clinical studies by securing ethical approvals in just 4 to 6 weeks, significantly reducing waiting periods compared to the median time of 234 days for site activation in clinical studies. This efficiency allows individuals to participate in paid clinical studies more quickly.
What advantages does Colombia's healthcare system offer for clinical studies?
Colombia's healthcare system is rated among the best globally and offers notable cost reductions exceeding 30% compared to North America and Western Europe, ensuring quality and affordability for participants in paid clinical studies.
How does Centricity Research enhance participation in clinical studies?
Centricity Research provides access to innovative therapies not yet available to the public and offers financial compensation for participation, with payments ranging from $75 to $4,500 depending on the study. This combination makes participation appealing to many individuals.
What role do financial incentives play in clinical studies?
Financial incentives are critical in motivating participation in paid clinical studies. They ensure that individuals are informed about the associated risks and benefits, making the opportunity more attractive.
What distinguishes Mayo Clinic's approach to clinical trials?
Mayo Clinic is committed to high-quality clinical research, emphasizing stringent protocols and comprehensive oversight to ensure reliable outcomes. This dedication fosters trust among participants, assuring them of their health and safety during studies.
Why is trust important in clinical research?
Trust is essential for participant engagement and retention in clinical studies. It requires continuous effort to maintain, as it significantly impacts the reliability of study results and the overall advancement of medical knowledge.
What initiatives are being taken to improve clinical research in Latin America?
The collaboration between bioaccess™ and Caribbean Health Group aims to position Barranquilla as a premier research hub in Latin America, enhancing research management services and positively impacting local economies through job creation and healthcare improvements.
What is the current participation rate in research studies?
Only 5% of eligible patients engage in research studies, highlighting the challenges in attracting individuals and the importance of building trust throughout the research process.