


Establishing a solid budget and contract management framework is essential for the success of clinical research, especially for Croatian investigators who must navigate the complexities of funding and regulatory requirements. By implementing best practices in budgeting and contract oversight, researchers can optimize their resources and enhance the integrity of their studies. However, many face significant challenges in accurately forecasting expenses and managing contracts effectively.
How can investigators ensure they are adequately prepared to tackle these obstacles while maximizing their project’s potential? This question is critical as it sets the stage for understanding the intricate landscape of clinical research funding and compliance.
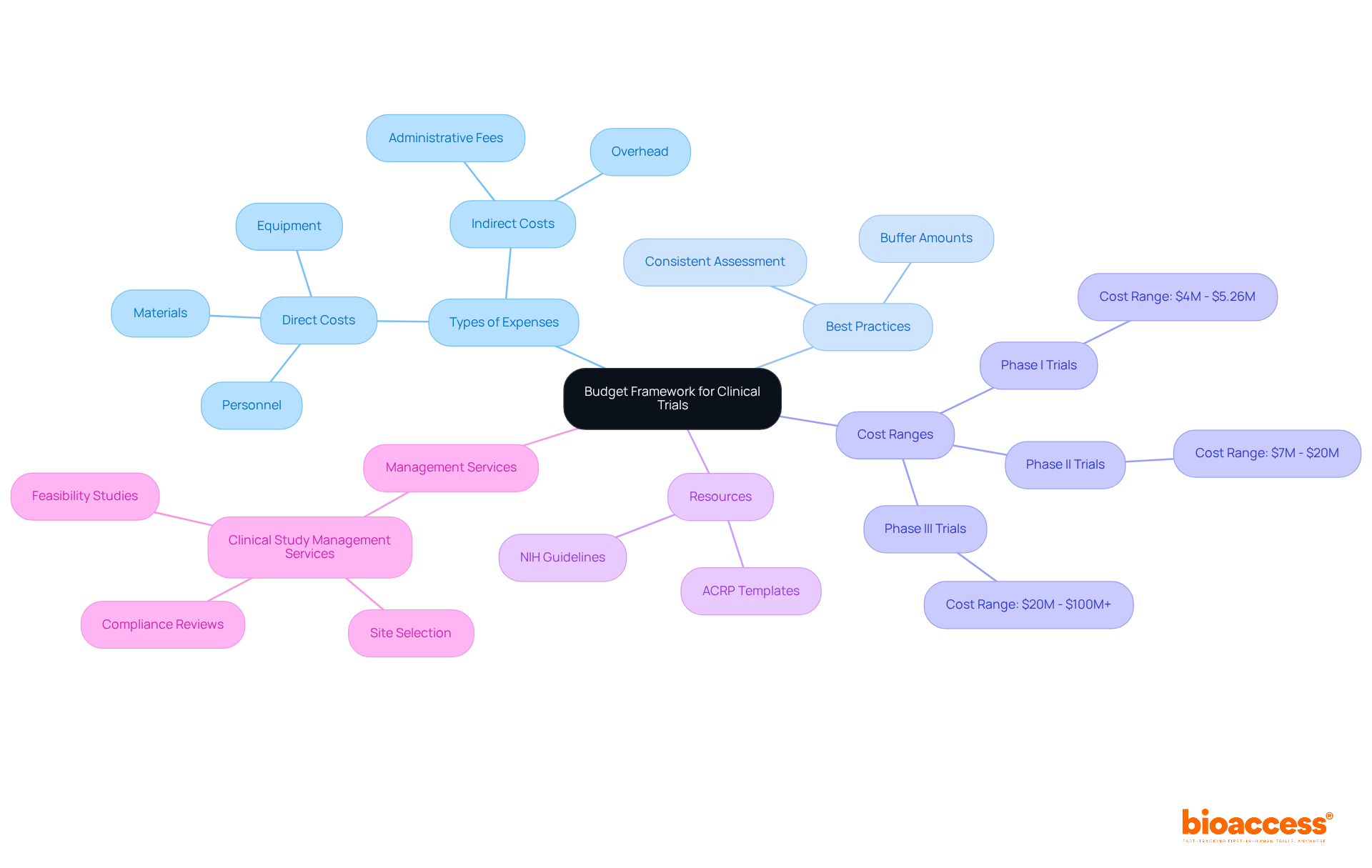
To establish a robust framework for budget and contract management for Croatian investigators, researchers must first identify all potential expenses related to the clinical trial. This encompasses direct costs like personnel, equipment, and materials, as well as indirect expenditures such as overhead and administrative fees. Each expense should be backed by a detailed explanation of its necessity for the project. For example, if a specific piece of equipment is vital for data collection, the financial plan must include a justification for its inclusion, clearly linking it to the study's objectives.
Including buffer amounts for key financial categories is considered an industry best practice in budget and contract management for Croatian investigators to manage unforeseen expenses, ensuring financial oversight throughout the study. Additionally, referencing average costs associated with various phases of clinical trials can provide valuable context. For instance:
Moreover, utilizing templates and guidelines from established organizations like the NIH and ACRP can significantly enhance the budget and contract management for Croatian investigators. These resources offer insights into common pitfalls, such as site startup delays and screen failure reimbursements, helping investigators avoid overlooking essential financial items. Consistently assessing and revising the financial plan throughout the project lifecycle to accommodate unexpected costs or changes in project scope is crucial for maintaining project integrity.
Furthermore, employing comprehensive clinical study management services, such as those provided by bioaccess, can streamline the budgeting process. Their expertise in feasibility studies, site selection, compliance reviews, setup, import permits, project management, and reporting ensures that all aspects of the evaluation are accounted for, ultimately leading to a more accurate and effective budget framework.
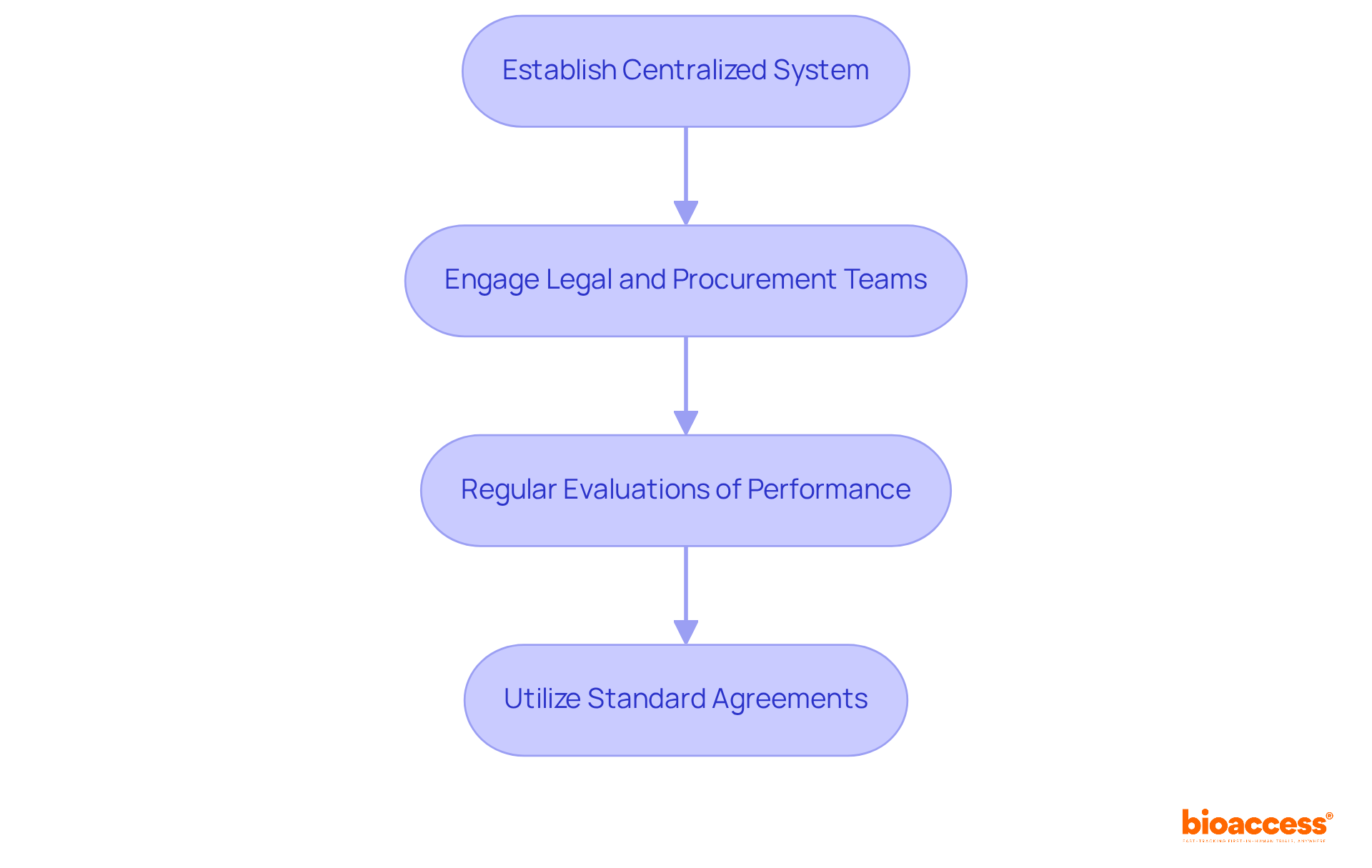
To execute efficient budget and contract management for Croatian investigators, it is necessary to establish a centralized system for monitoring all arrangements related to clinical studies. This system should encompass essential details such as agreement terms, renewal dates, and performance metrics. By employing agreement management software, organizations can significantly enhance visibility and streamline communication among stakeholders. This is particularly vital given that nearly 75% of clinical trials are conducted by research organizations (CROs), highlighting the critical need for effective agreement processes.
Engaging legal and procurement teams early in the agreement negotiation process is essential to ensure alignment with organizational policies and regulatory requirements. Regular evaluations of performance against established key performance indicators (KPIs) can uncover areas for improvement and ensure compliance with obligations. For instance, tracking the average duration required to finalize agreements can identify bottlenecks, enabling timely interventions. Research shows that utilizing standard agreements can drastically reduce negotiation time, cutting it from an average of 73 days to just 39 days. This underscores the importance of streamlined processes in enhancing operational efficiency.
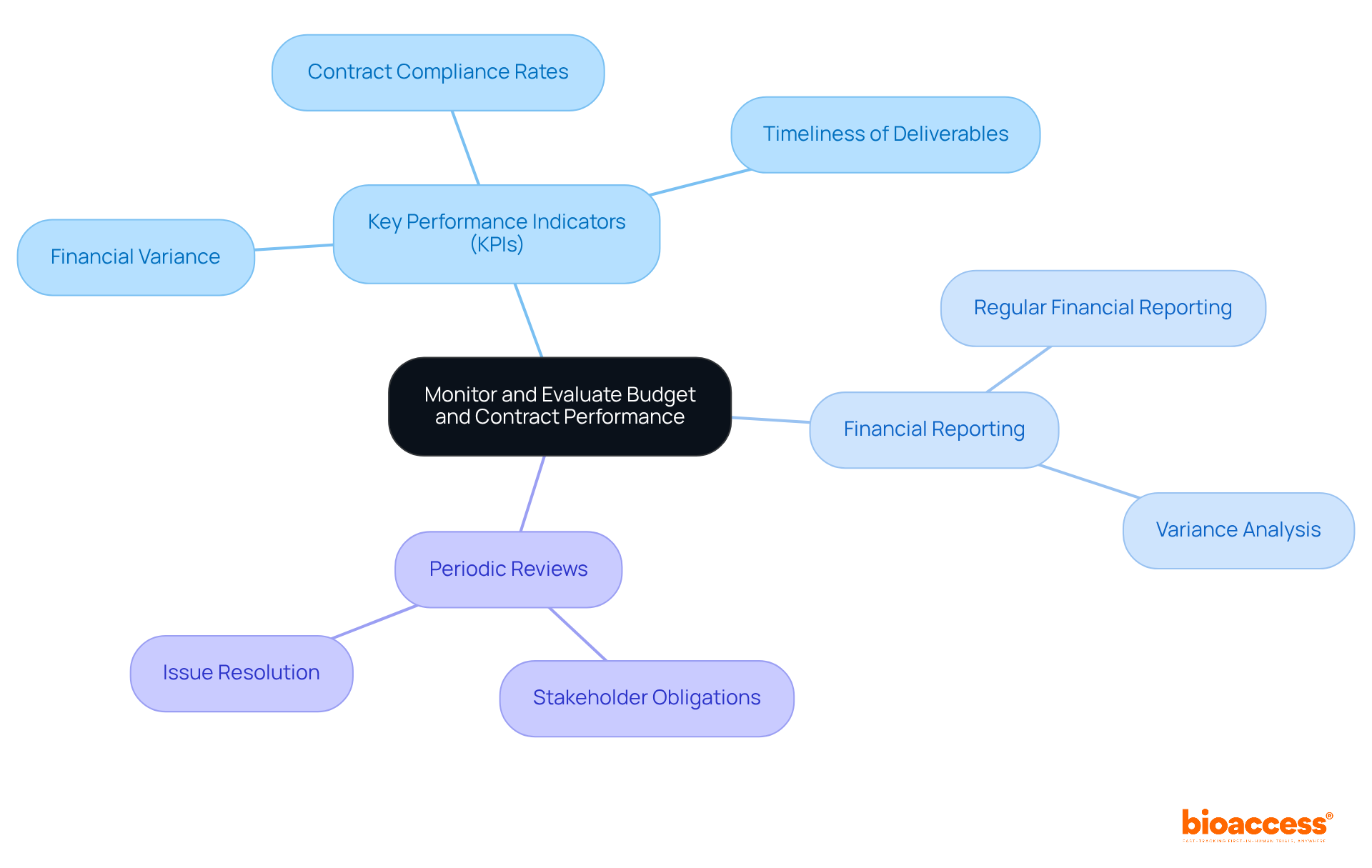
To effectively oversee and assess budget and contract management for Croatian investigators in clinical research, it is essential to establish a set of key performance indicators (KPIs) that align with the project's objectives. These KPIs should encompass critical metrics related to budget and contract management for Croatian investigators, such as:
Regular financial reporting is essential for budget and contract management for Croatian investigators, enabling the comparison of actual expenditures against the financial plan. This process allows for the identification of any variances in the context of budget and contract management for Croatian investigators. For instance, if a specific line item exceeds its budget, a thorough analysis should be conducted to determine the cause and whether adjustments are necessary.
Furthermore, periodic reviews of budget and contract management for Croatian investigators are vital to ensure that all parties fulfill their obligations and that any issues are addressed promptly. This proactive approach not only enhances project efficiency but also fosters trust among stakeholders.
Establishing a comprehensive budget and contract management framework is essential for Croatian investigators involved in clinical research. By carefully identifying all potential expenses and implementing effective practices, researchers can ensure their financial plans are not only robust but also aligned with the project's objectives. This structured approach fosters transparency and accountability, ultimately enhancing the integrity of the research process.
Throughout this article, we've discussed key strategies, including the importance of:
Additionally, the value of continuous monitoring and evaluation through key performance indicators (KPIs) has been highlighted as a means to maintain financial oversight and compliance throughout the project lifecycle. These insights serve as vital tools for investigators aiming to navigate the complexities of budget and contract management effectively.
In light of these practices, it is imperative for researchers to adopt a proactive approach to budget and contract management. By prioritizing diligent planning, regular assessments, and leveraging available resources, Croatian investigators can not only mitigate risks but also enhance the overall success of their clinical trials. The significance of a well-structured budget framework cannot be overstated; it is a foundational element that supports the advancement of scientific research and the attainment of impactful results.
What is the first step in establishing a budget framework for clinical trials in Croatia?
The first step is to identify all potential expenses related to the clinical trial, including direct costs like personnel, equipment, and materials, as well as indirect expenditures such as overhead and administrative fees.
How should expenses be justified in the budget?
Each expense should be backed by a detailed explanation of its necessity for the project, linking it clearly to the study's objectives.
Why is it recommended to include buffer amounts in the budget?
Including buffer amounts for key financial categories is considered an industry best practice to manage unforeseen expenses, ensuring financial oversight throughout the study.
What are the typical costs associated with different phases of clinical trials?
Phase I trials typically range from $4 million to $5.26 million, Phase II trials can cost between $7 million and $20 million, and Phase III trials may require $20 million to over $100 million.
How can templates and guidelines from organizations like NIH and ACRP assist Croatian investigators?
These resources provide insights into common pitfalls, helping investigators avoid overlooking essential financial items and enhancing budget and contract management.
Why is it important to assess and revise the financial plan throughout the project lifecycle?
Consistently assessing and revising the financial plan is crucial for accommodating unexpected costs or changes in project scope, which helps maintain project integrity.
What role do comprehensive clinical study management services play in budgeting?
Services like those provided by bioaccess can streamline the budgeting process by ensuring that all aspects of the evaluation are accounted for, leading to a more accurate and effective budget framework.