


In the realm of clinical research, effective supply management stands as a cornerstone of successful trial execution, not merely a logistical necessity. In Croatia, where stringent regulations and a centralized healthcare system shape the landscape, mastering the intricacies of clinical trial supply management can significantly enhance operational efficiency and compliance. However, navigating the complexities of regulatory requirements, fierce competition, and logistical challenges presents a formidable task for stakeholders.
How can they leverage best practices to overcome these hurdles and ensure the success of their clinical trials in this evolving environment? This question is crucial as it prompts stakeholders to reflect on their own challenges and consider innovative solutions that can drive their success.
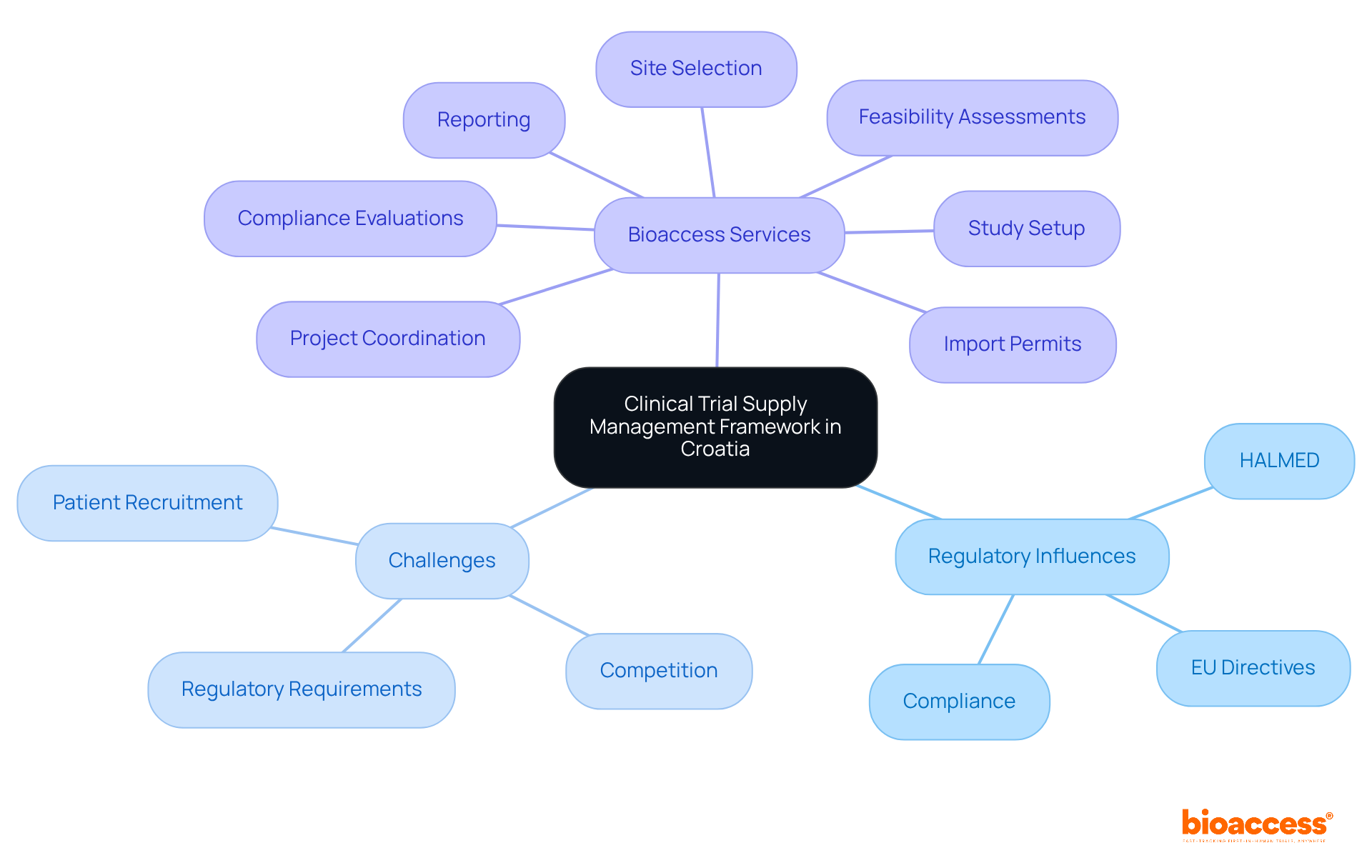
In Croatia, clinical trial supply management plays a pivotal role in shaping the landscape of clinical research. Influenced by national regulations and European Union directives, particularly the Ordinance on Clinical Trials on Medicinal Products and the EU Clinical Trials Regulation (CTR), this framework standardizes processes across member states. As a result, it enhances compliance and operational efficiency in research studies, making it crucial for stakeholders in the field.
At the heart of this framework is the Croatian Agency for Medicinal Products and Medical Devices (HALMED). This agency supervises the authorization of research studies and investigational medicinal products (IMPs), ensuring that all studies adhere to ethical and regulatory standards. HALMED's oversight is essential for safeguarding participant rights and safety, reinforcing the integrity of clinical research in Croatia.
However, medical device startups in Croatia encounter several challenges. These include:
Despite these hurdles, Croatia's centralized healthcare system facilitates effective communication and oversight of research studies, positioning the country as an attractive location for research activities.
To navigate these challenges effectively, bioaccess offers extensive research study oversight services. These services encompass:
By understanding the CTSM framework and leveraging bioaccess's tailored solutions, stakeholders can adeptly manage the complexities of clinical trial supply management in Croatia. This ensures that all necessary approvals and documentation are meticulously prepared before study initiation. Such a proactive approach not only streamlines the process but also significantly enhances the likelihood of successful study outcomes, particularly with the full applicability of the Clinical Trials Regulation (EU) No 536/2014 commencing in January 2025.
The success of clinical research relies heavily on effective clinical trial supply management in Croatia. This process hinges on the meticulous gathering and maintenance of essential documentation, which includes several key components:
Clinical Trial Application (CTA): This document encompasses the cover letter, application form, study protocol, and Investigator's Brochure (IB). The CTA must be submitted to HALMED for approval before any study can commence. Typically, the approval procedure for standard Investigational Medicinal Products (IMPs) lasts between 60 to 106 days, depending on the complexity of the study and the thoroughness of the documentation provided.
Ethics Committee Approval: Securing consent from a local ethics committee is vital to ensure that the study adheres to ethical standards and protects participant rights. The Central Ethics Committee (CEC) generally reviews applications within 30 to 90 days. Proactive communication can significantly mitigate potential delays in this process.
Informed Consent Forms (ICFs): These documents must be carefully prepared and approved to ensure that participants are fully informed about the study and consent to participate. Clarity and comprehensiveness in ICFs are essential for ethical compliance and participant understanding.
Import Licenses: For studies involving imported investigational products, acquiring the necessary licenses is essential. This process can take up to 15 working days, highlighting the importance of timely planning.
By ensuring that all documentation is complete and compliant with local regulations, sponsors can facilitate a smoother process and significantly reduce the risk of delays. A thorough understanding of the regulatory environment and strict adherence to compliance standards are essential for improving the success rates of research studies in Croatia.

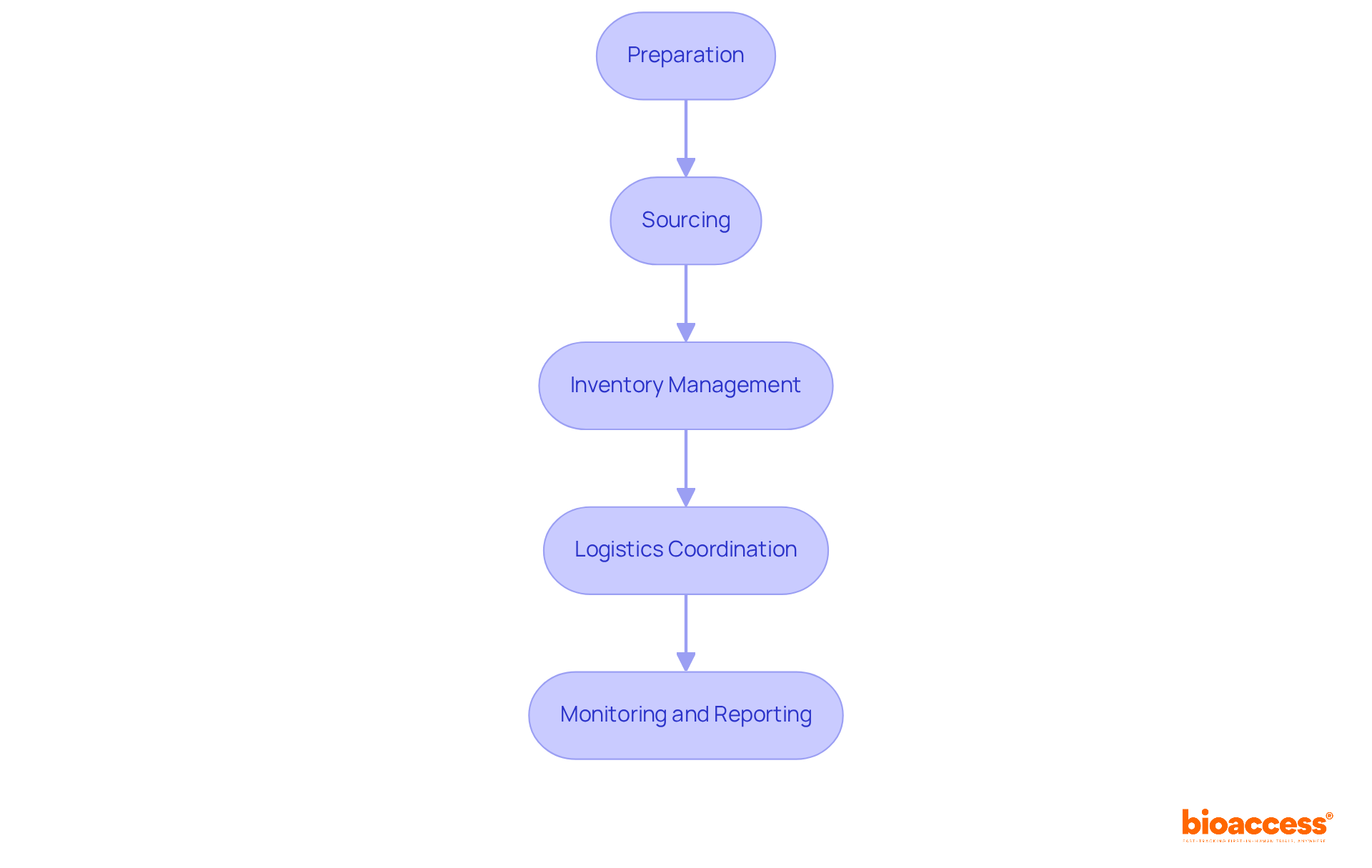
To ensure efficient clinical trial supply management in Croatia, it’s crucial to follow these key steps:
Preparation: Begin by evaluating the experiment's needs, including the categories and amounts of materials required. Collaborate with clinical teams to fully understand the protocol and patient requirements. Effective planning is essential for minimizing delays - approximately 80% of clinical studies face challenges in participant recruitment, often exacerbated by logistical issues.
Sourcing: Identify reliable vendors for investigational products and additional materials. Establish contracts that clearly define delivery timelines, quality standards, and compliance requirements. Case studies show that successful sourcing strategies can significantly boost trial efficiency, especially in regions with strong supplier networks.
Inventory Management: Implement a robust inventory management system to track supplies, monitor expiration dates, and manage stock levels. Regular audits are vital to ensure accuracy in clinical trial supply management in Croatia, as incomplete documentation accounts for 28% of delayed submissions in the Clinical Trial Application (CTA) process.
Logistics Coordination: Develop a logistics strategy that covers transportation methods, storage conditions (particularly for temperature-sensitive products), and distribution timelines to medical sites. Integrating advanced technologies, such as IoT for real-time tracking, can enhance logistics efficiency and compliance, addressing the complexities of modern research trials.
Monitoring and Reporting: Continuously monitor inventory levels and delivery timelines while maintaining open communication with vendors and healthcare locations. Establish a reporting system to address any issues swiftly, as proactive oversight can mitigate risks associated with resource interruptions.
By adhering to this structured approach, clinical study teams can enhance their operational efficiency and reduce the risk of supply-related disruptions, ultimately contributing to the success of their clinical research initiatives.
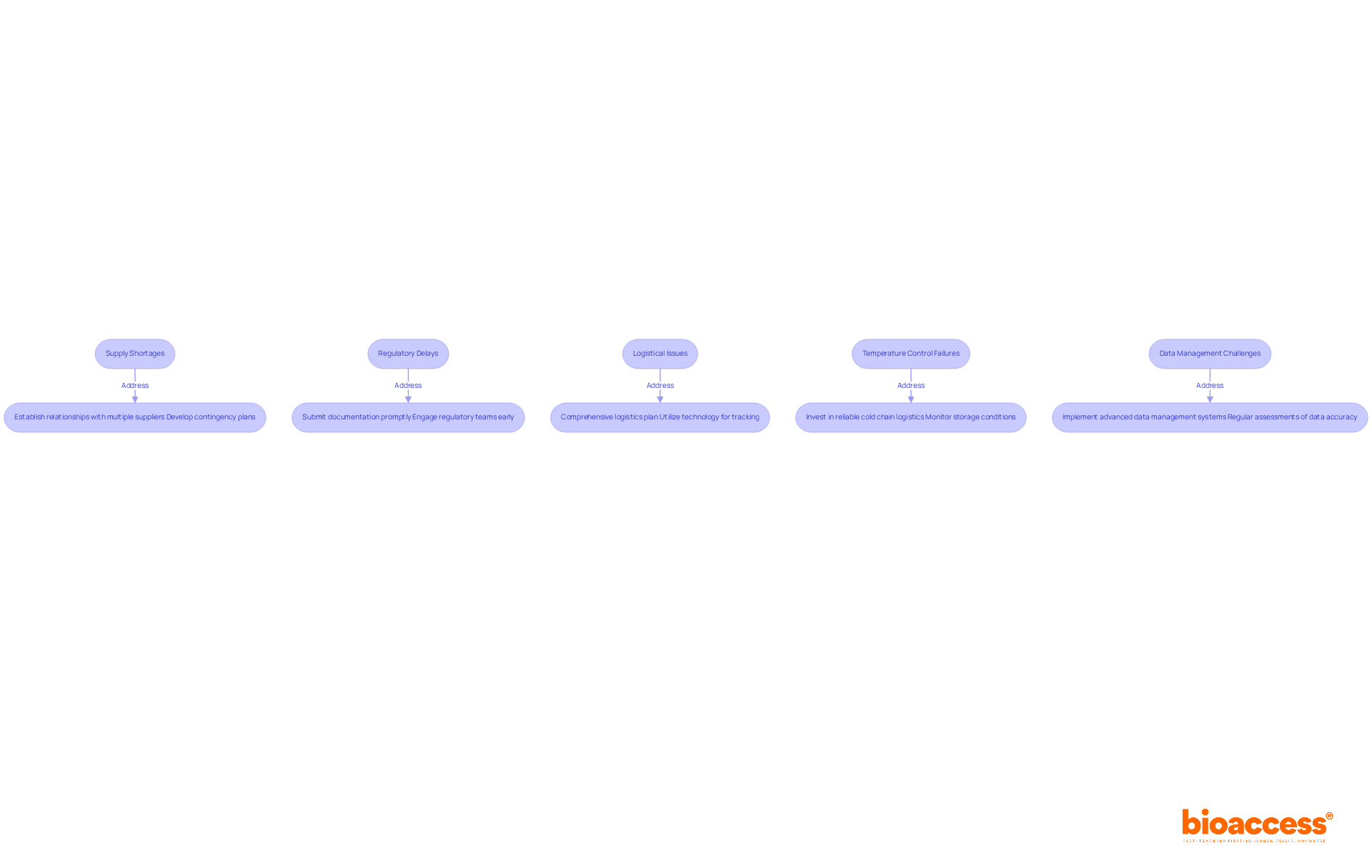
Clinical trial resource coordination presents significant challenges that demand strategic solutions. Understanding these issues is crucial for enhancing operational efficiency in clinical research. Here are key challenges and effective strategies to address them:
Supply Shortages: To anticipate potential shortages, it’s vital to establish robust relationships with multiple suppliers. Developing contingency plans that incorporate alternative sourcing options can greatly minimize risks associated with distribution disruptions. Recent surveys reveal that 63% of institutions face monthly drug shortages, underscoring the importance of proactive logistics oversight.
Regulatory Delays: Staying informed about regulatory changes is essential. Ensure that all documentation is submitted promptly and maintain regular communication with regulatory bodies to address any concerns early. Engaging regulatory teams early in the process can prevent common missteps that lead to delays in approvals.
Logistical Issues: A comprehensive logistics plan is indispensable. This should include backup transportation options and contingency measures for unexpected delays. Utilizing technology for real-time shipment tracking can enhance visibility and responsiveness, which is critical for maintaining project timelines.
Temperature Control Failures: For temperature-sensitive products, investing in reliable cold chain logistics solutions is necessary. Continuously monitoring storage conditions and implementing a system for immediate reporting and response to temperature excursions will safeguard product integrity.
Data Management Challenges: Advanced data management systems are crucial for tracking inventory, monitoring supply levels, and generating compliance reports. Regular assessments of data accuracy can help mitigate risks linked to logistical inefficiencies, which often contribute to research delays.
By proactively addressing these challenges, clinical trial teams can bolster their operational resilience and ensure the successful execution of their trials through effective clinical trial supply management in Croatia.
Mastering clinical trial supply management in Croatia is not just important; it's essential for cultivating a thriving research environment. The framework established by national regulations and EU directives standardizes processes, enhancing compliance and operational efficiency. Stakeholders must adeptly navigate this landscape to ensure their clinical trials are conducted ethically and successfully.
Key insights from the article highlight the significance of:
Challenges such as regulatory delays, supply shortages, and logistical issues can hinder progress. However, proactive strategies - including establishing robust vendor relationships and leveraging advanced data management systems - can significantly mitigate these risks.
Ultimately, the success of clinical trials in Croatia relies on a proactive and informed approach to supply management. By embracing best practices and tackling common challenges head-on, stakeholders can enhance their operational efficiency and contribute to the advancement of clinical research in the region. This commitment to excellence in clinical trial supply management is vital for ensuring the integrity and success of research initiatives, paving the way for innovative medical solutions.
What is the importance of clinical trial supply management in Croatia?
Clinical trial supply management is crucial in Croatia as it shapes the landscape of clinical research, enhancing compliance and operational efficiency through standardized processes influenced by national regulations and EU directives.
Which regulatory bodies oversee clinical trials in Croatia?
The Croatian Agency for Medicinal Products and Medical Devices (HALMED) supervises the authorization of research studies and investigational medicinal products (IMPs), ensuring adherence to ethical and regulatory standards.
What are the primary challenges faced by medical device startups in Croatia?
Medical device startups in Croatia face challenges such as convoluted regulatory requirements, fierce competition from established players, and difficulties in patient recruitment.
How does Croatia's healthcare system benefit clinical research?
Croatia's centralized healthcare system facilitates effective communication and oversight of research studies, making the country an attractive location for research activities.
What services does bioaccess offer to help navigate clinical trial supply management challenges?
Bioaccess offers services including feasibility assessments, site selection, compliance evaluations, study setup, import permits, project coordination, and reporting.
Why is it important to understand the clinical trial supply management framework?
Understanding the CTSM framework helps stakeholders manage the complexities of clinical trial supply management effectively, ensuring all necessary approvals and documentation are prepared before study initiation.
What is the significance of the Clinical Trials Regulation (EU) No 536/2014 for clinical trials in Croatia?
The full applicability of the Clinical Trials Regulation (EU) No 536/2014, commencing in January 2025, will enhance the regulatory landscape and streamline processes for clinical trials in Croatia.