


The landscape of drug development in Australia is evolving rapidly, with feasibility studies emerging as pivotal assessments that determine the viability of clinical trials. These evaluations not only assess critical factors like patient availability and site capacities but also streamline the research process, ultimately enhancing the chances of successful outcomes.
As the biopharmaceutical sector grapples with increasing complexities, a pressing question arises: how can researchers effectively navigate the intricate web of regulatory requirements and stakeholder expectations? Addressing this challenge is essential to ensure that feasibility planning leads to successful drug studies.
Feasibility planning for drug studies in Australia includes essential evaluations that assess the practicality and viability of proposed clinical experiments. These investigations meticulously examine factors such as:
By evaluating the likelihood of successful trial execution, feasibility analyses significantly reduce risks and optimize resource allocation.
In Australia, where the regulatory environment is notably supportive, feasibility planning for drug studies amplifies the importance of these research efforts, ensuring that innovative therapies can progress effectively through the development pipeline. Recent insights reveal that around 25% of investigational new drug applications include critical data from outside the U.S., highlighting the growing reliance on international sites for clinical research. Furthermore, successful viability assessments have demonstrated their impact; for instance, a thorough examination in an oncology trial enabled recruitment to be completed three months ahead of schedule, showcasing the effectiveness of strategic site selection.
bioaccess® offers a comprehensive process for advancing medical device evaluations, which includes:
As the biopharmaceutical sector faces increasing pressures, the significance of thorough feasibility planning for drug studies in Australia cannot be overstated, as it lays the groundwork for successful studies and ultimately enhances patient outcomes.
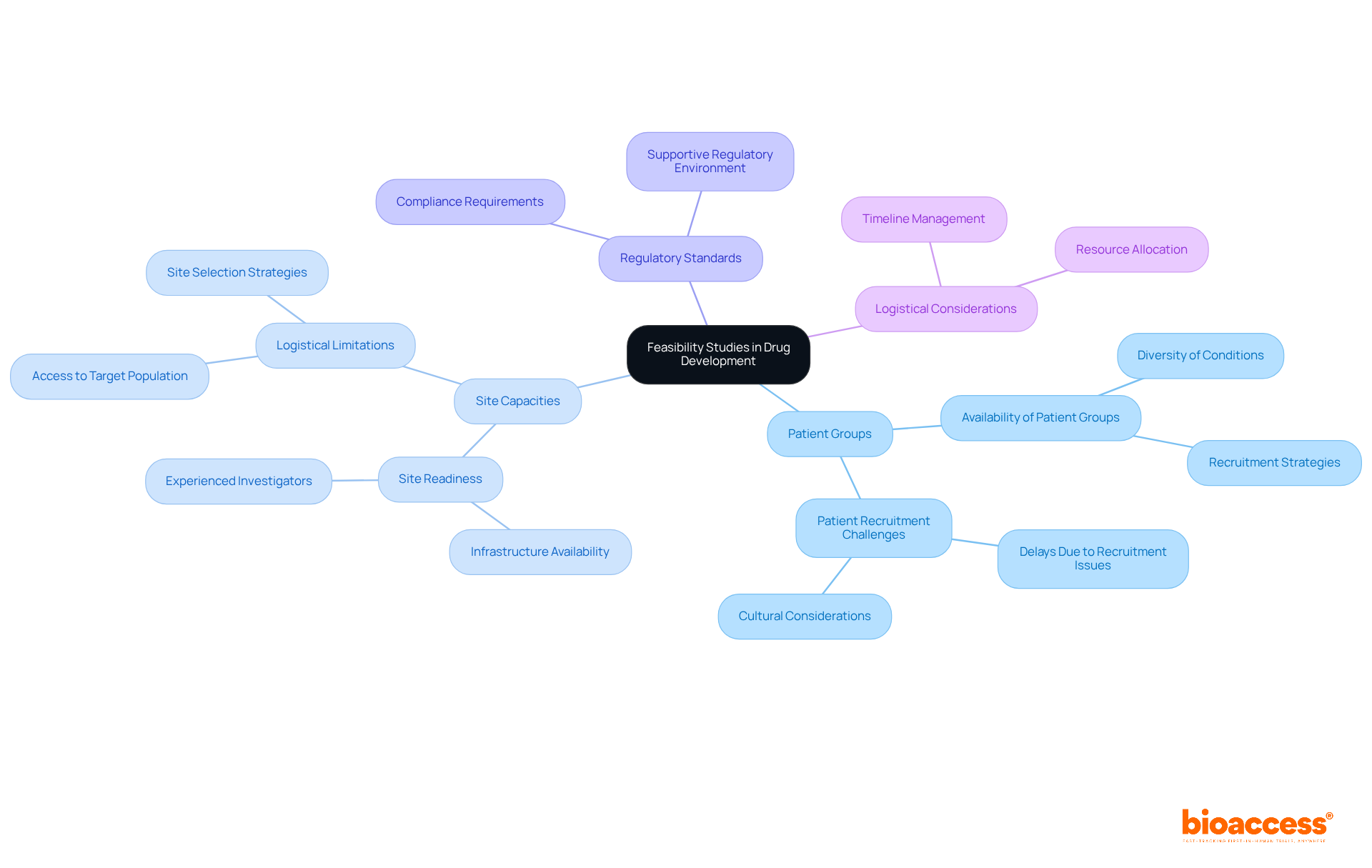
Feasibility assessments are vital for determining the success of clinical experiments. They encompass essential components such as:
Efficient site choice is paramount; facilities with a proven track record in similar assessments significantly boost recruitment rates and enhance data quality.
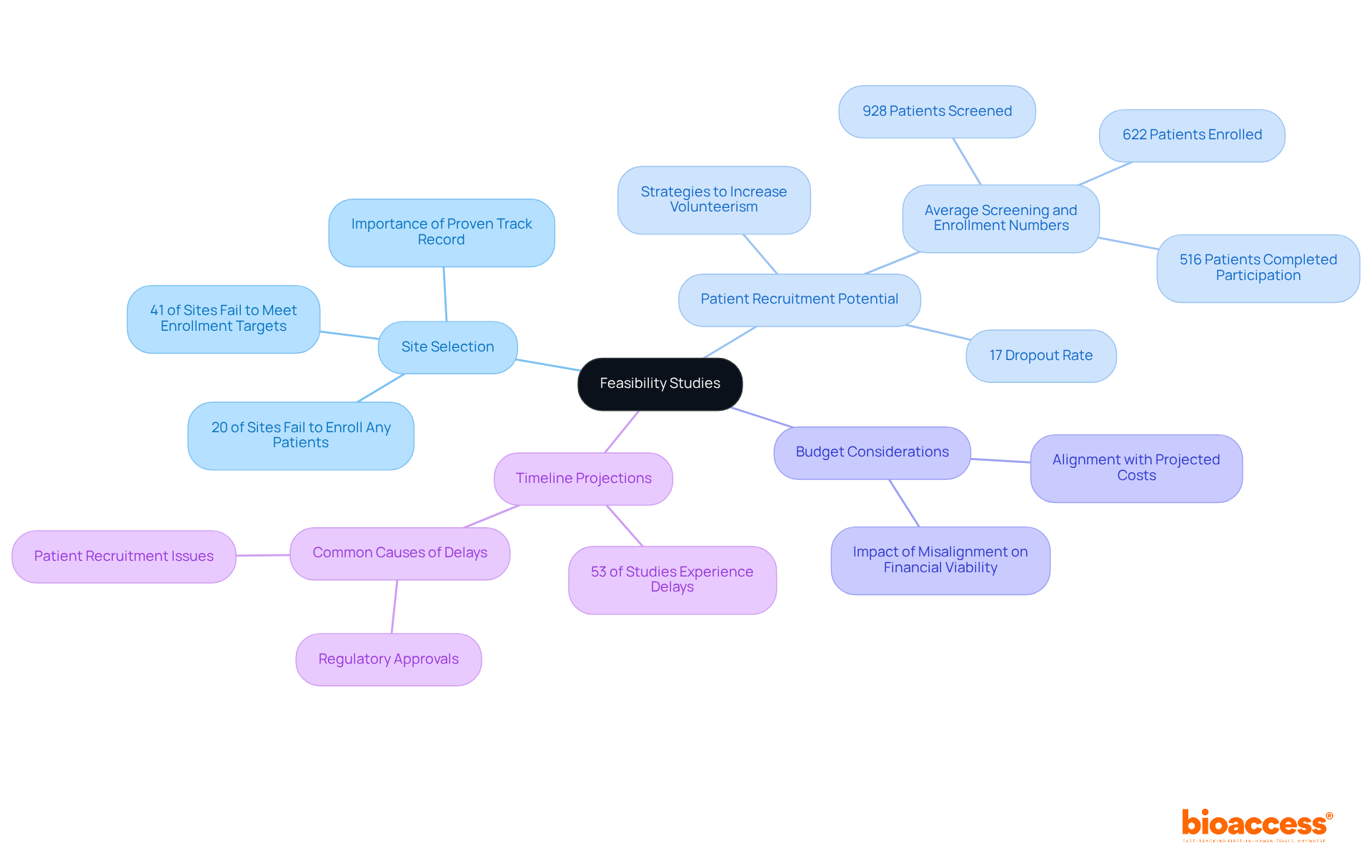
Statistics reveal that 41% of activated investigative sites fail to meet their target enrollment numbers. This underscores the critical importance of selecting sites with strong past performances and adequate infrastructure. Additionally, understanding budgetary implications is essential, as misalignment with projected costs can jeopardize financial viability. Practical timeline forecasts are equally crucial; research indicates that nearly 53% of tests experience prolonged timelines, often due to delays in regulatory approvals or patient recruitment.
By prioritizing these elements, research directors can significantly improve the feasibility planning for drug studies in Australia and enhance the success of drug trials. Collaboration and strategic planning are key to navigating these challenges effectively.
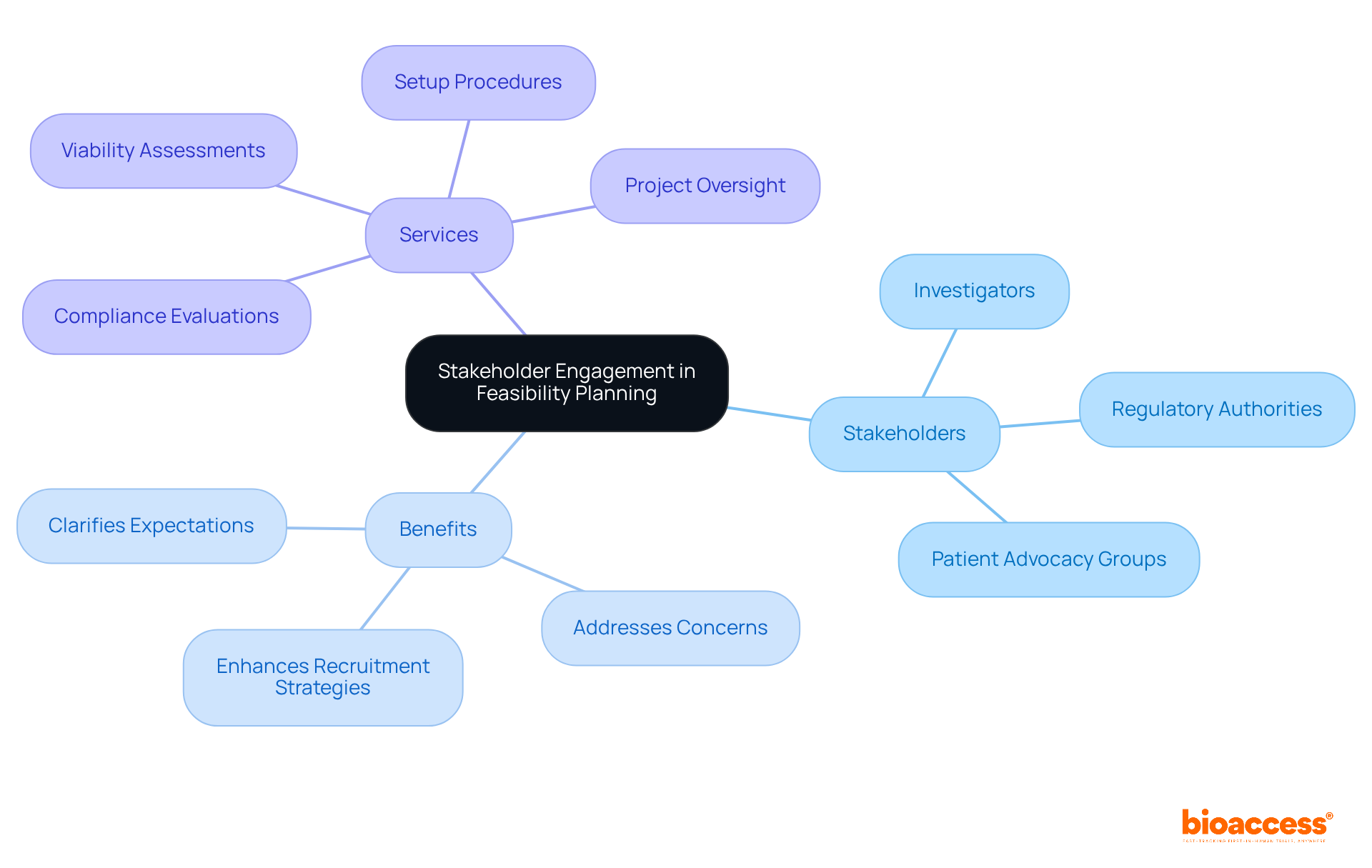
Effective stakeholder engagement is essential in the clinical trial process and is a critical aspect of feasibility planning for drug studies in Australia, requiring collaboration with investigators, regulatory authorities, and patient advocacy groups. Early involvement of these stakeholders not only clarifies expectations but also addresses concerns and gathers insights that can significantly impact feasibility planning for drug studies in Australia. For instance, including patient representatives offers invaluable perspectives on recruitment strategies and research design, ultimately enhancing participant engagement.
Consistent communication and regular updates during the evaluation process foster trust and transparency-elements that are crucial for successful collaboration. This approach not only increases the likelihood of meeting recruitment goals but also supports the feasibility planning for drug studies in Australia by aligning the research with the needs and preferences of the patient population, thereby improving the overall success rates of medical studies. Furthermore, bioaccess® provides comprehensive management services for research studies, including:
These services further enhance productive stakeholder involvement.
By acknowledging common challenges, such as neglecting the concerns of patient representatives, stakeholders can refine the planning process. Ultimately, effective stakeholder involvement, supported by bioaccess's services, is expected to enhance the feasibility planning for drug studies in Australia, benefiting both researchers and patients alike.
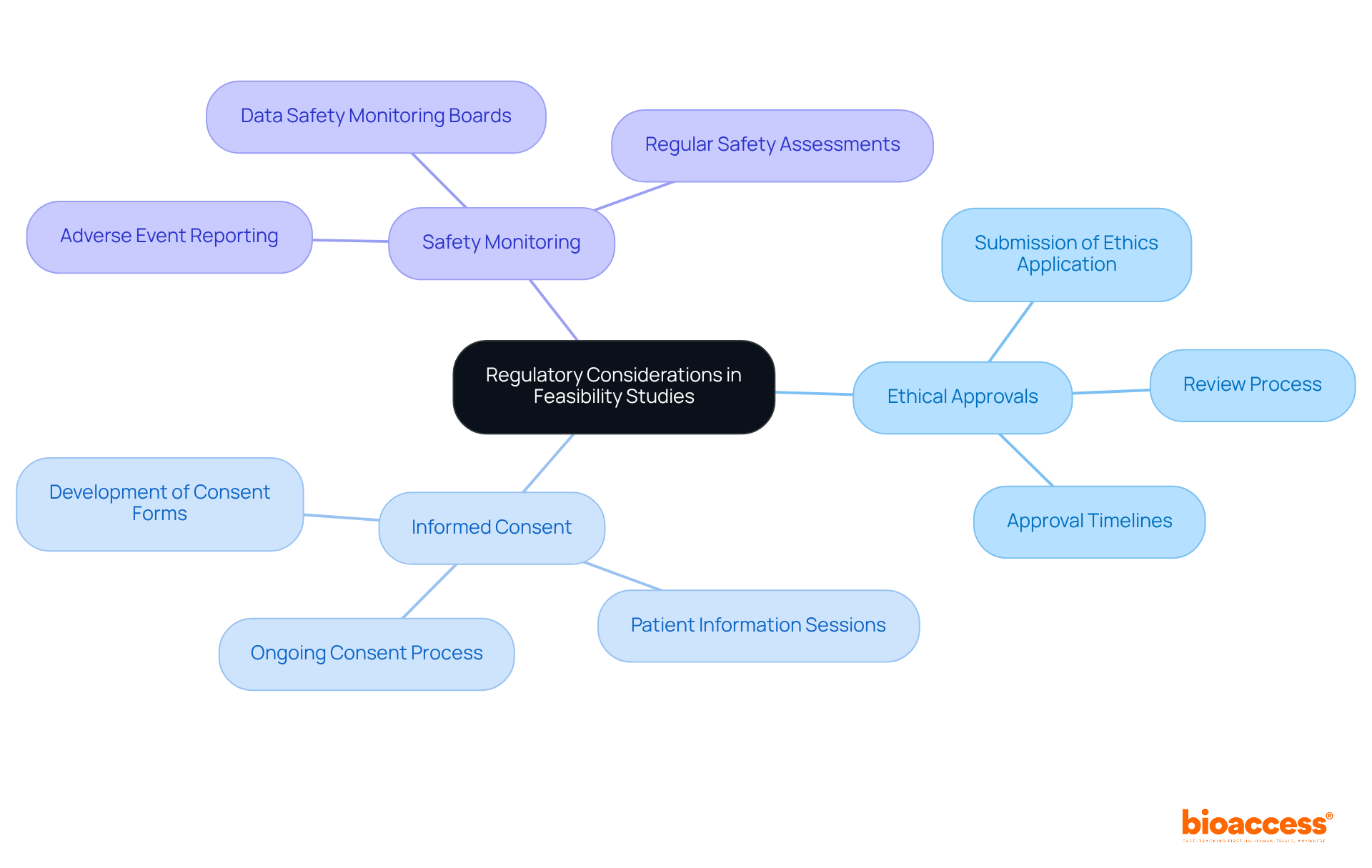
Incorporating regulatory considerations into viability studies is crucial for ensuring compliance with both local and international guidelines. In Australia, researchers must navigate the Therapeutic Goods Administration (TGA) regulations, which govern the approval of medical studies. This involves a thorough understanding of requirements for:
By embedding these regulatory aspects into the feasibility planning for drug studies in Australia, researchers can early identify potential hurdles and devise strategies to address them. This proactive approach not only enhances the likelihood of regulatory approval but also bolsters the overall integrity and success of clinical trials.
The significance of feasibility planning for drug studies in Australia is paramount. By thoroughly evaluating factors such as patient availability, site capacities, and regulatory standards, researchers can accurately assess the viability of their clinical trials. This strategic approach minimizes risks and optimizes resource allocation, ultimately facilitating the advancement of innovative therapies through the development pipeline.
Key insights from the article underscore the necessity of comprehensive feasibility assessments, which include:
Engaging stakeholders early in the process - such as patient representatives and regulatory authorities - fosters collaboration and ensures that research aligns with the needs of the patient population. Moreover, integrating regulatory considerations into feasibility studies aids in navigating compliance requirements, thereby enhancing the overall integrity and success of clinical trials.
In light of these findings, it is evident that effective feasibility planning is crucial for the progression of drug studies in Australia. By prioritizing thorough evaluations and involving all relevant stakeholders, researchers can significantly improve the success rates of clinical trials and, ultimately, enhance patient outcomes. Embracing these best practices not only fortifies the research landscape but also reinforces the commitment to delivering innovative and effective therapies to those in need.
What are feasibility studies in drug development?
Feasibility studies in drug development assess the practicality and viability of proposed clinical experiments, evaluating factors such as suitable patient groups, site capacities, regulatory standards, and logistical considerations.
Why are feasibility studies important in drug development?
Feasibility studies are important as they evaluate the likelihood of successful trial execution, significantly reduce risks, and optimize resource allocation for clinical trials.
How does the regulatory environment in Australia affect feasibility studies?
Australia has a notably supportive regulatory environment, which amplifies the importance of feasibility planning for drug studies, ensuring that innovative therapies can progress effectively through the development pipeline.
What percentage of investigational new drug applications include data from outside the U.S.?
Around 25% of investigational new drug applications include critical data from outside the U.S., indicating a growing reliance on international sites for clinical research.
Can you provide an example of the impact of successful feasibility assessments?
A thorough examination in an oncology trial allowed recruitment to be completed three months ahead of schedule, demonstrating the effectiveness of strategic site selection.
What services does bioaccess® offer for medical device evaluations?
bioaccess® offers services that include assessing viability, selecting research locations and lead investigators, reviewing research documents for compliance with national regulations, and providing updates on project status, inventory, and adverse occurrences.
Why is thorough feasibility planning emphasized in the biopharmaceutical sector?
Thorough feasibility planning is emphasized as it lays the groundwork for successful studies and ultimately enhances patient outcomes, especially in the face of increasing pressures within the biopharmaceutical sector.