


In the realm of clinical trials, informed consent is a cornerstone of ethical research. It ensures that participants are not only aware of potential risks and benefits but are also empowered to make informed decisions about their involvement. In Montenegro, adhering to informed consent guidelines transcends mere regulatory obligation; it represents a commitment to safeguarding individual rights and enhancing the integrity of clinical research.
However, as researchers navigate the complexities of these guidelines, they often face significant challenges. How can they ensure that consent processes are both compliant and comprehensible to diverse participant populations? Exploring effective strategies and best practices for informed consent in Montenegro not only illuminates the legal landscape but also highlights the ethical imperatives that underpin successful clinical trials.
Obtaining consent is a critical and evolving procedure that ensures individuals fully understand the characteristics of a clinical trial, including its aim, methods, risks, and advantages. This process transcends the mere act of signing a form; it embodies an ongoing dialogue between researchers and participants. Such engagement is essential for respecting individual autonomy, empowering individuals to make informed decisions about their involvement. In Montenegro, the informed consent guidelines for trials in Montenegro are not merely a legal obligation but a moral imperative that safeguards individuals' rights and well-being.
A comprehensive understanding of informed consent guidelines for trials in Montenegro is essential for researchers to comply with local regulations and foster trust with participants. At Bioaccess, we emphasize the integration of informed consent within our clinical trial management services. This includes compliance assessments and trial preparation, ensuring that every aspect is handled with the utmost care and adherence to regulatory standards. By prioritizing informed consent, we not only meet legal requirements but also uphold ethical standards that protect participants and enhance the integrity of clinical research.
In Montenegro, the informed consent guidelines for trials in Montenegro are firmly established in the Law on Medicines and the Law on Clinical Trials. These regulations mandate that the informed consent guidelines for trials in Montenegro must be obtained before any individual participates in a clinical trial. The necessity of transparency and the autonomy of participants is further underscored by ethical guidelines, particularly in relation to the informed consent guidelines for trials in Montenegro. Researchers are responsible for ensuring that consent forms comply with informed consent guidelines for trials in Montenegro, ensuring they are clear, comprehensive, and presented in a language that is easily understood by individuals. Ethical review boards play a crucial role in overseeing the consent process according to the informed consent guidelines for trials in Montenegro, guaranteeing that participants are fully informed and that their rights are safeguarded throughout the study.
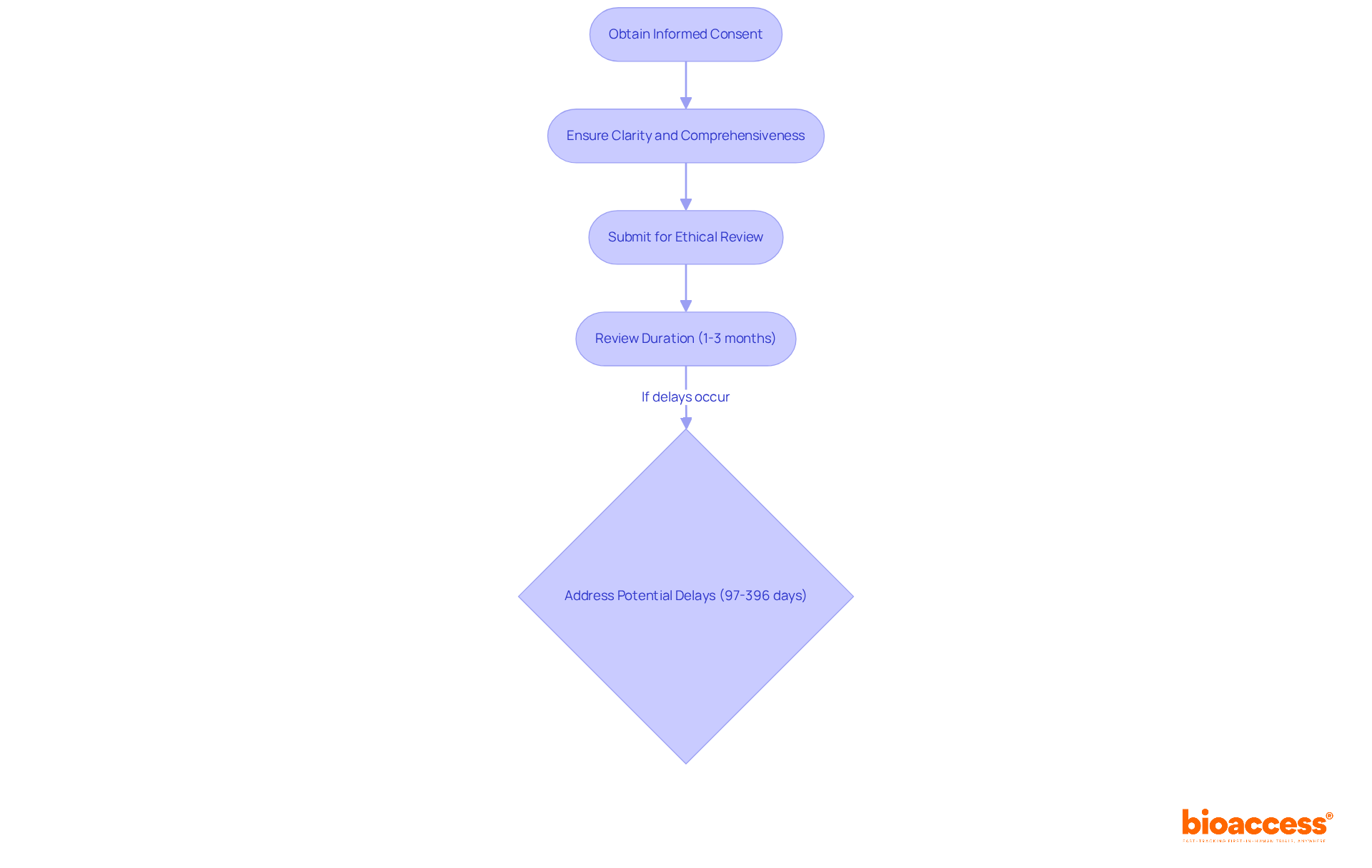
The average duration for the ethical review of drug trials typically spans from 1 to 3 months, highlighting the importance of timely submissions. Adherence to Good Clinical Practice (GCP) and the principles outlined in the Declaration of Helsinki is vital for upholding ethical standards in clinical trials. Researchers must also remain cognizant of potential delays in the ethical assessment submission process, which can range from 97 to 396 days due to incomplete documentation.
To streamline this process, bioaccess provides a comprehensive suite of clinical trial management services, including:
This structured approach to obtaining approval is essential for maintaining ethical standards and fostering trust in clinical research. By collaborating with bioaccess, stakeholders can navigate the complexities of clinical trials more effectively, ensuring that ethical considerations are prioritized.
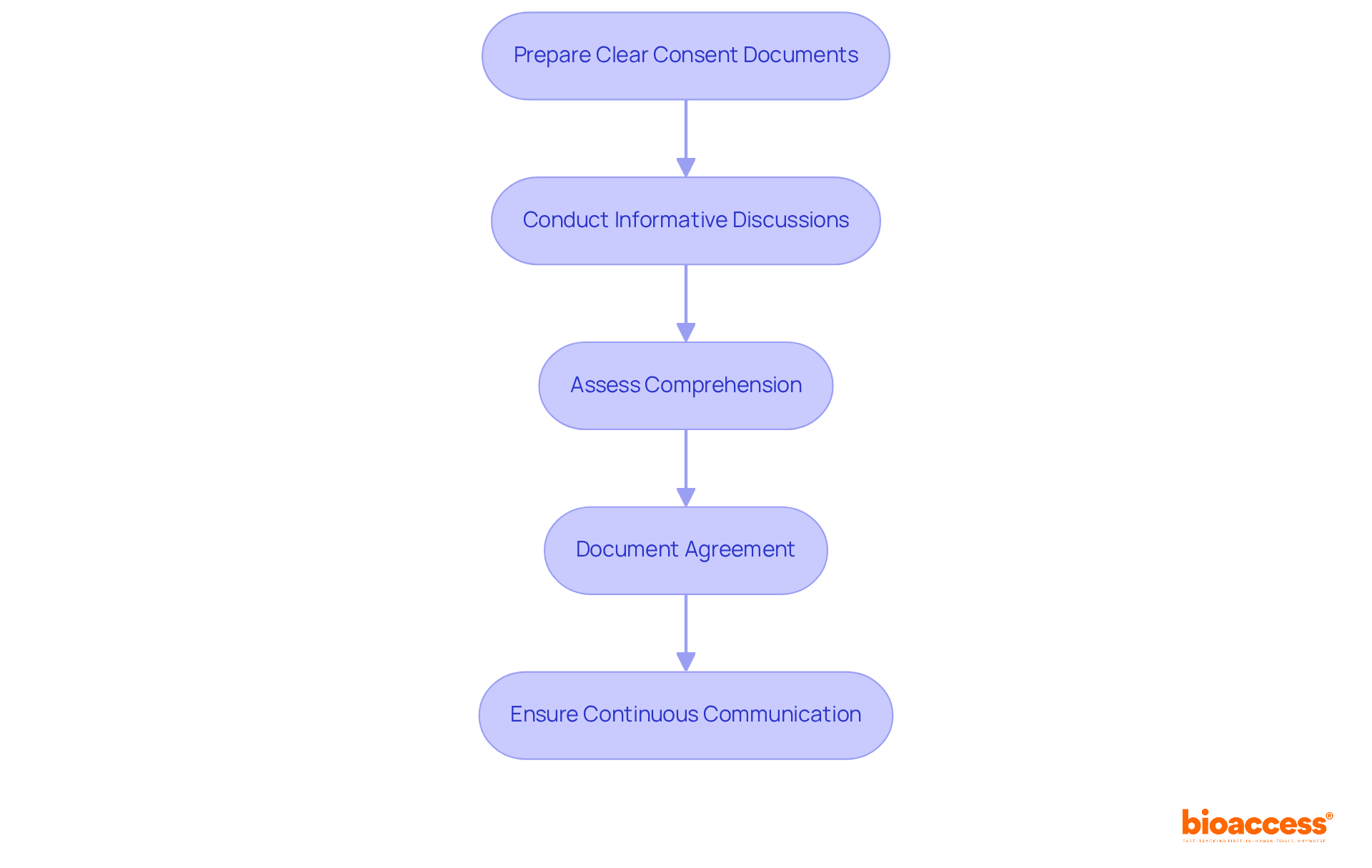
To implement effective informed consent procedures, researchers must follow these essential steps:
Prepare Clear Consent Documents: Consent forms should be crafted in plain language, avoiding technical jargon. They must thoroughly address all crucial details regarding the study, including potential risks, benefits, and individuals' rights to withdraw.
Conduct Informative Discussions: Engage participants in meaningful conversations about the study, encouraging them to ask questions and express concerns. This dialogue is vital for ensuring that individuals fully understand the study's implications.
Assess Comprehension: After providing information, evaluate participants' understanding through open-ended questions. This assessment is crucial to confirm that they grasp the significance of their involvement.
Document Agreement: Secure written approval and meticulously document the entire process. This documentation serves as a legal record and demonstrates adherence to ethical standards.
Ensure Continuous Communication: Keep participants updated throughout the study, especially if new details arise that may affect their choice to proceed.
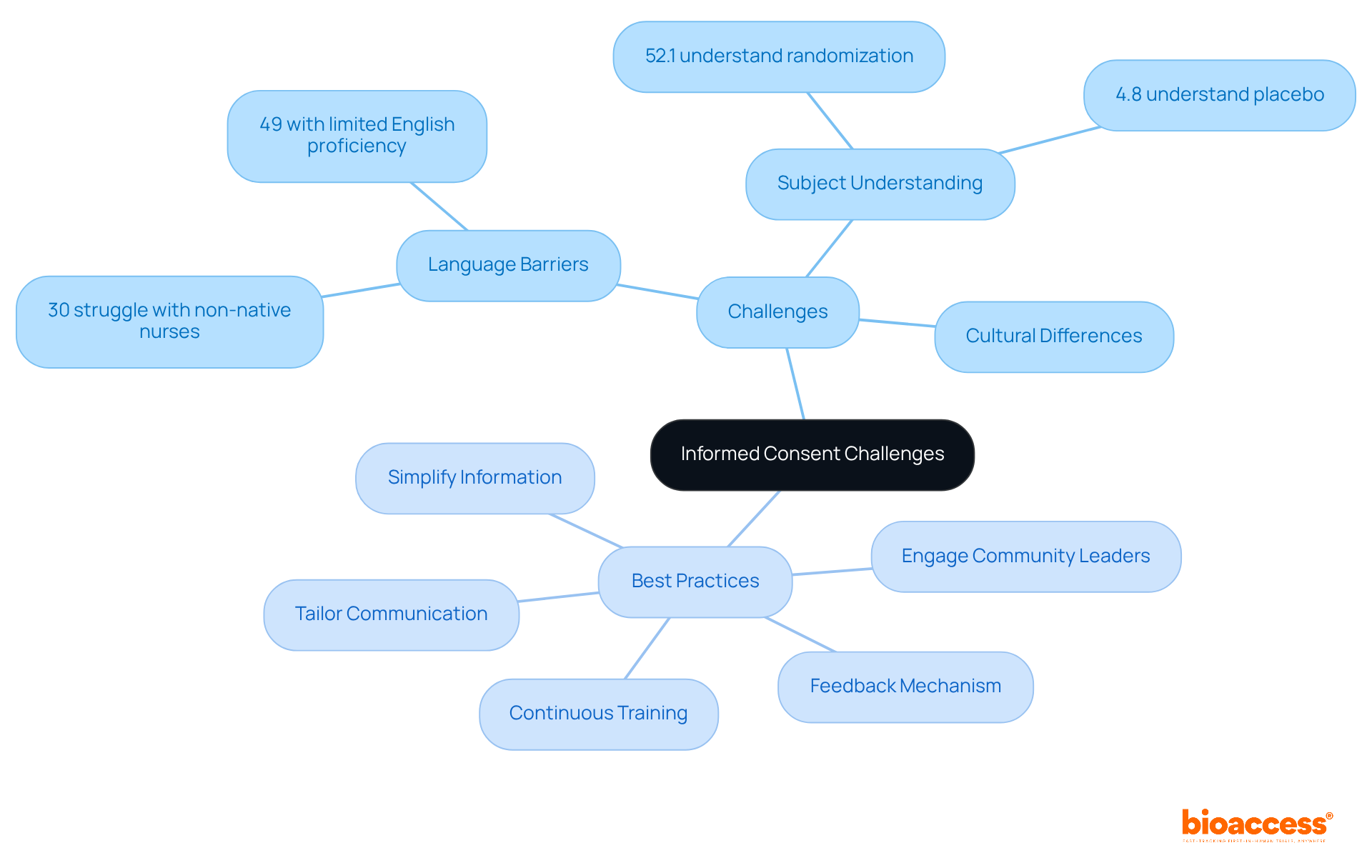
Statistics reveal that knowledge of informed consent elements varies significantly among individuals, with comprehension rates ranging from 52.1% to 75.8%. For example, 67.0% of participants understood potential risks and side effects, while only 4.8% grasped the concept of placebo. These figures underscore the necessity for clear communication strategies.
Effective consent documents often utilize visual aids and summaries to enhance clarity. The i-CONSENT initiative has developed informed consent guidelines for trials in Montenegro that emphasize the importance of customizing information for the audience, ensuring that consent procedures are inclusive and accessible. By employing these strategies, researchers can foster a more knowledgeable and engaged participant group, ultimately enhancing the ethical integrity of clinical trials.
Acquiring knowledgeable approval in clinical trials presents significant challenges, particularly regarding language barriers, subject understanding, and cultural differences. Statistics reveal that language obstacles can severely impact the informed consent process; for instance, 30% of patients attended to by non-native speaking nurses struggle to comprehend medical instructions. Furthermore, 49% of patients with limited English proficiency report difficulties in understanding their medical situations. To effectively navigate these challenges, researchers should adopt the following best practices:
Tailor Communication: Adapt the consent process to align with the cultural and linguistic backgrounds of participants. Utilizing interpreters can greatly enhance comprehension and ensure that attendees fully grasp the study's details.
Simplify Information: Break down complex information into manageable segments. Employ visuals or analogies to clarify challenging concepts, making the consent process more accessible.
Engage Community Leaders: Collaborate with local community leaders to build trust and facilitate open discussions about the study. Their involvement can significantly boost engagement and willingness to participate.
Continuous Training: Provide ongoing education for research personnel focused on effective communication and ethical considerations in the consent process. This ensures that all team members are well-equipped to meet the diverse needs of participants.
Feedback Mechanism: Establish a system for participants to voice their concerns or suggestions regarding the consent process. This feedback is invaluable for refining future trials and enhancing overall participant satisfaction.
By implementing these best practices, researchers can markedly improve the informed consent guidelines for trials in Montenegro, ensuring that individuals are fully informed and engaged. Additionally, recognizing that only 52.1% of participants understand the concept of randomization in trials highlights the critical need for these strategies to enhance comprehension and uphold ethical standards in clinical research.
Informed consent in clinical trials is not just a formality; it is a crucial process that guarantees participants are fully aware of what their involvement entails. This tutorial underscores the importance of understanding the informed consent guidelines specific to Montenegro, highlighting that these guidelines serve as both legal requirements and ethical obligations that safeguard participants' rights and well-being.
Key insights from the article reveal the necessity for clear communication, the pivotal role of ethical review boards, and the importance of customizing consent procedures to accommodate diverse participant backgrounds. By implementing effective strategies - such as preparing clear documents, engaging in meaningful discussions, and continuously assessing comprehension - researchers can cultivate an environment of trust and transparency. Challenges in the informed consent process, including language barriers and cultural differences, can be effectively addressed through best practices that prioritize participant understanding and engagement.
Ultimately, the significance of informed consent in clinical research cannot be overstated. Researchers must prioritize ethical considerations and participant autonomy, ensuring that individuals are not only informed but also empowered to make decisions regarding their participation. By mastering the informed consent guidelines for trials in Montenegro, stakeholders can enhance the integrity of clinical research and uphold the highest ethical standards, paving the way for successful and trustworthy trials.
What is informed consent in clinical trials?
Informed consent is a critical procedure that ensures individuals fully understand the characteristics of a clinical trial, including its aim, methods, risks, and advantages. It involves an ongoing dialogue between researchers and participants, rather than just signing a form.
Why is informed consent important in clinical trials?
Informed consent is important because it respects individual autonomy and empowers individuals to make informed decisions about their involvement in a trial. It safeguards individuals' rights and well-being.
What are the informed consent guidelines for trials in Montenegro?
The informed consent guidelines in Montenegro are not only a legal obligation but also a moral imperative that ensures the protection of individuals' rights and well-being during clinical trials.
How does Bioaccess approach informed consent in clinical trials?
Bioaccess emphasizes the integration of informed consent within their clinical trial management services, including compliance assessments and trial preparation, to ensure adherence to regulatory standards and ethical practices.
What is the significance of prioritizing informed consent in clinical research?
Prioritizing informed consent helps meet legal requirements while upholding ethical standards that protect participants and enhance the integrity of clinical research.