


Managing protocol deviations in TGA-regulated trials is not just a regulatory obligation; it’s a critical factor that can determine the success or failure of clinical research. These deviations, whether minor or serious, can jeopardize data integrity, participant safety, and ultimately, the credibility of study outcomes. As researchers navigate the complexities of compliance and reporting, the challenge lies in effectively identifying, documenting, and mitigating these discrepancies.
How can clinical trial teams ensure they are not only compliant but also safeguarding the integrity of their research in the face of potential protocol deviations? This question is paramount as it highlights the need for vigilance and proactive measures in clinical research.
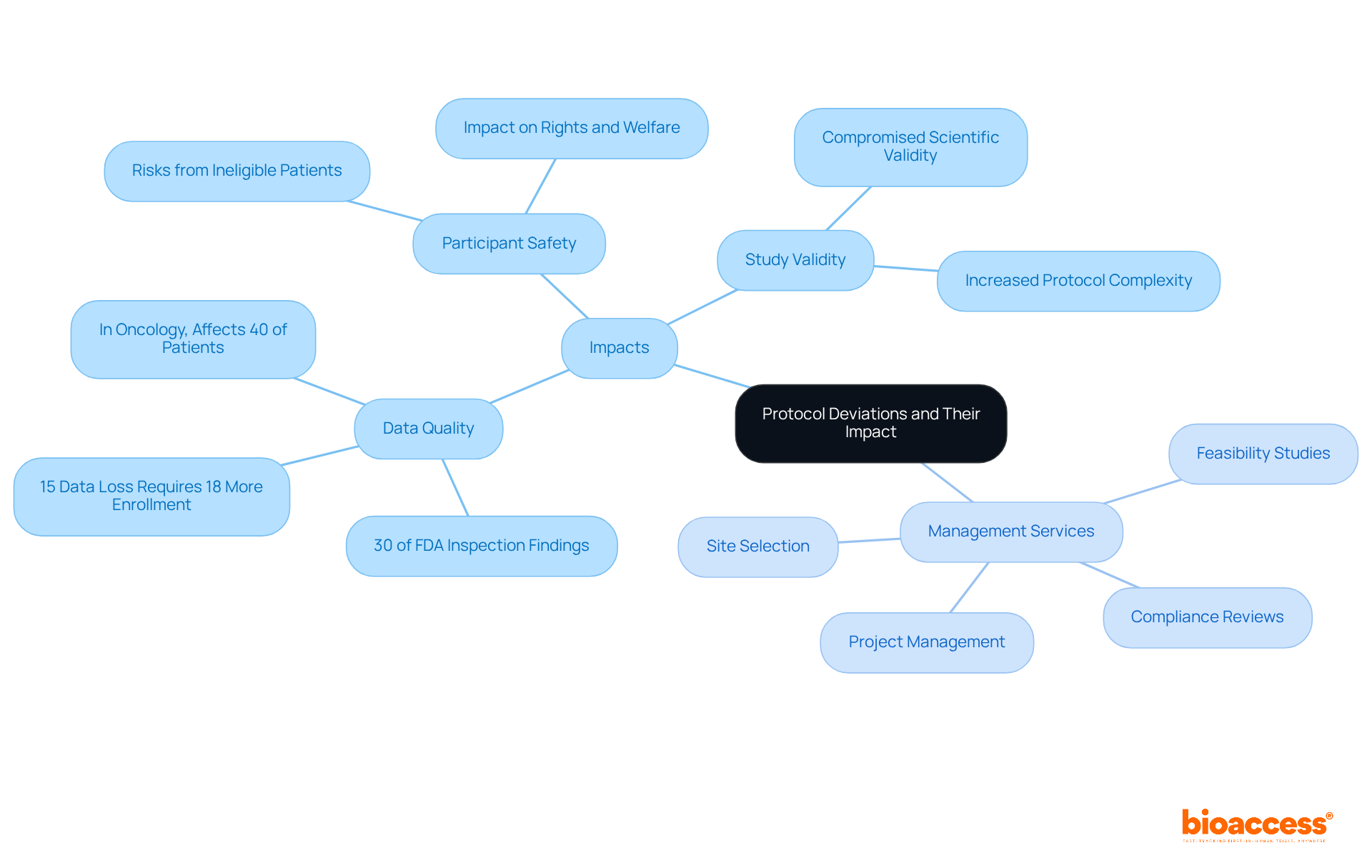
A protocol variation signifies any alteration or deviation from the study design or procedures outlined in the approved clinical research document. Such variations can occur either unintentionally or deliberately, and they can significantly impact the integrity of the trial. Understanding the implications of protocol variations is crucial, as they can affect data quality, participant safety, and the overall validity of study results. For instance, while a minor variation may not pose immediate risks, repeated occurrences can lead to serious issues in data interpretation and regulatory compliance. Therefore, managing protocol deviations in TGA-regulated trials is essential for identifying and maintaining the reliability of clinical research.
At bioaccess®, we provide comprehensive clinical trial management services, including:
Our expertise ensures that protocol issues are effectively addressed, safeguarding the integrity of your clinical trials. By collaborating with us, you can navigate the complexities of clinical research with confidence, ensuring that your studies meet the highest standards of quality and compliance.
Protocol deviations can be classified into several categories, each with distinct implications for clinical trials:
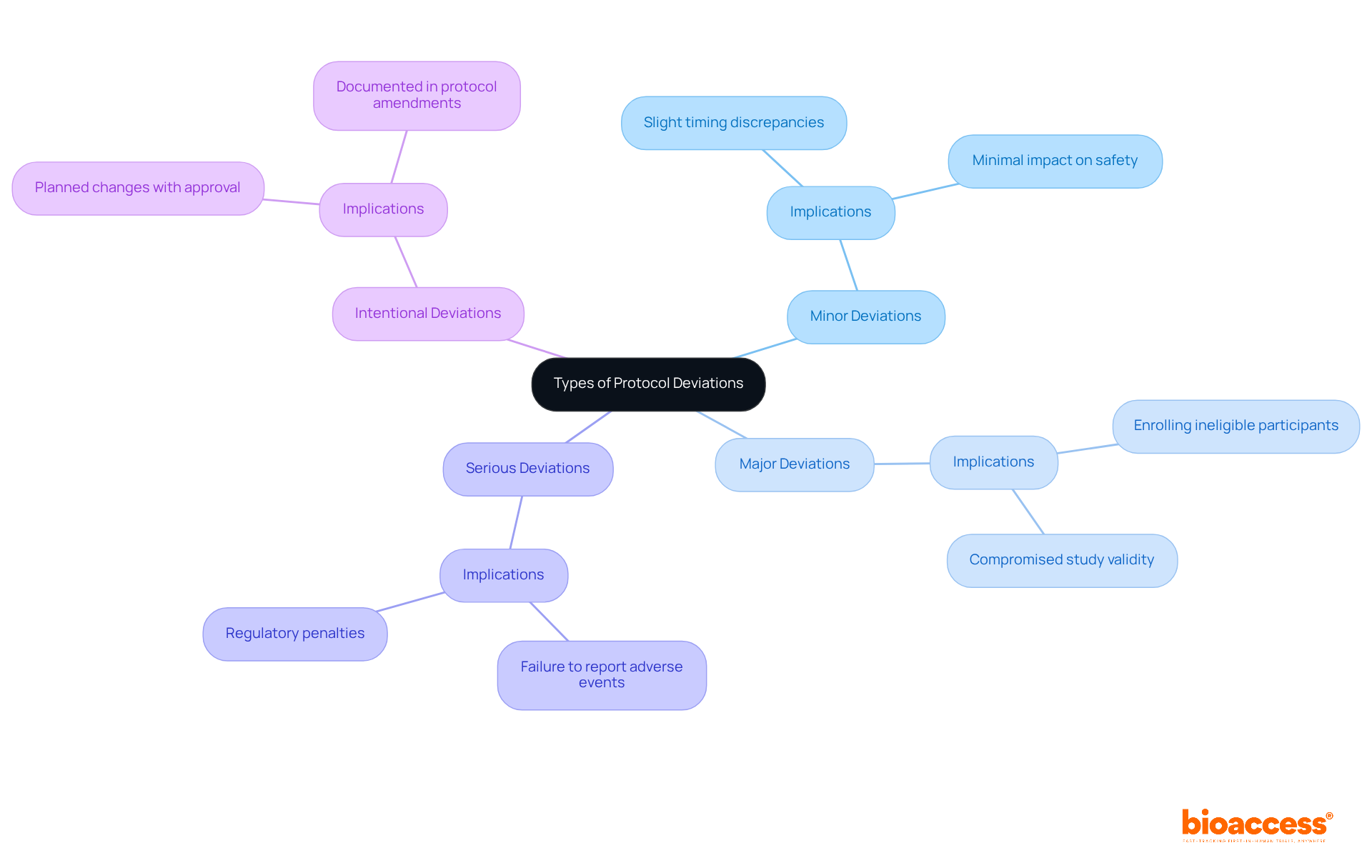
Minor Deviations: These deviations do not significantly impact participant safety or data integrity. For instance, slight timing discrepancies in follow-up visits may not affect the overall study outcomes.
Major Deviations: These can have serious consequences for participant safety or data integrity. Enrolling a participant who does not meet the eligibility criteria can compromise the validity of the study results and potentially endanger the participant's well-being.
Serious Deviations: These are significant breaches that necessitate immediate reporting to regulatory bodies. A failure to report adverse events, for example, can lead to regulatory penalties and jeopardize the trial's integrity.
Intentional Deviations: These are planned changes made with prior approval, often documented in protocol amendments. Such variations may be necessary to adjust to unexpected situations while ensuring adherence to regulatory standards.
Understanding these classifications is essential for researchers, as it allows them to evaluate the seriousness of discrepancies and respond appropriately. Recent research indicates that small variations contribute to a considerable share of reported problems, while major discrepancies, although less common, can have substantial effects on results. Experts emphasize the significance of thorough training and compliance with guidelines to reduce both types of inconsistencies, thereby improving the overall quality and dependability of clinical research.
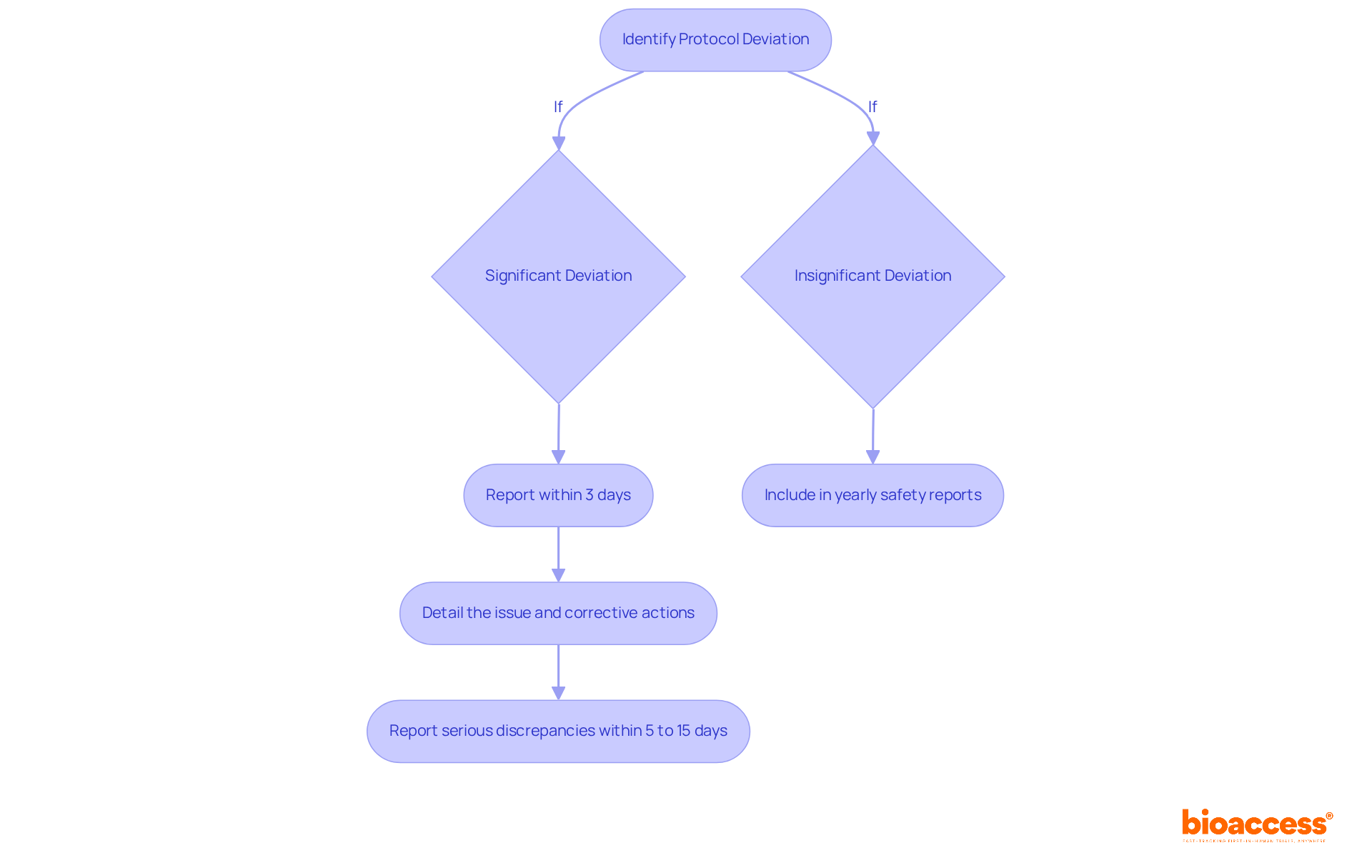
In TGA-regulated trials, managing protocol deviations in TGA-regulated trials is crucial for adhering to specific reporting requirements for protocol changes. Researchers must report significant variations to the TGA within 3 calendar days of identification. This reporting includes a detailed account of the issue's nature, its potential impact on participant safety, and any corrective actions implemented. Serious discrepancies require reporting within 5 to 15 days. While slight variations can be included in yearly safety reports, significant discrepancies necessitate prompt reporting to maintain study integrity. Understanding these necessities, including the categorization of variances into significant and insignificant groups, is vital for managing protocol deviations in TGA-regulated trials while upholding regulations and ensuring the successful execution of clinical studies.
At Bioaccess, we provide comprehensive clinical trial management services that encompass:
We ensure that all aspects of TGA compliance are meticulously handled, reinforcing our commitment to excellence in clinical research.
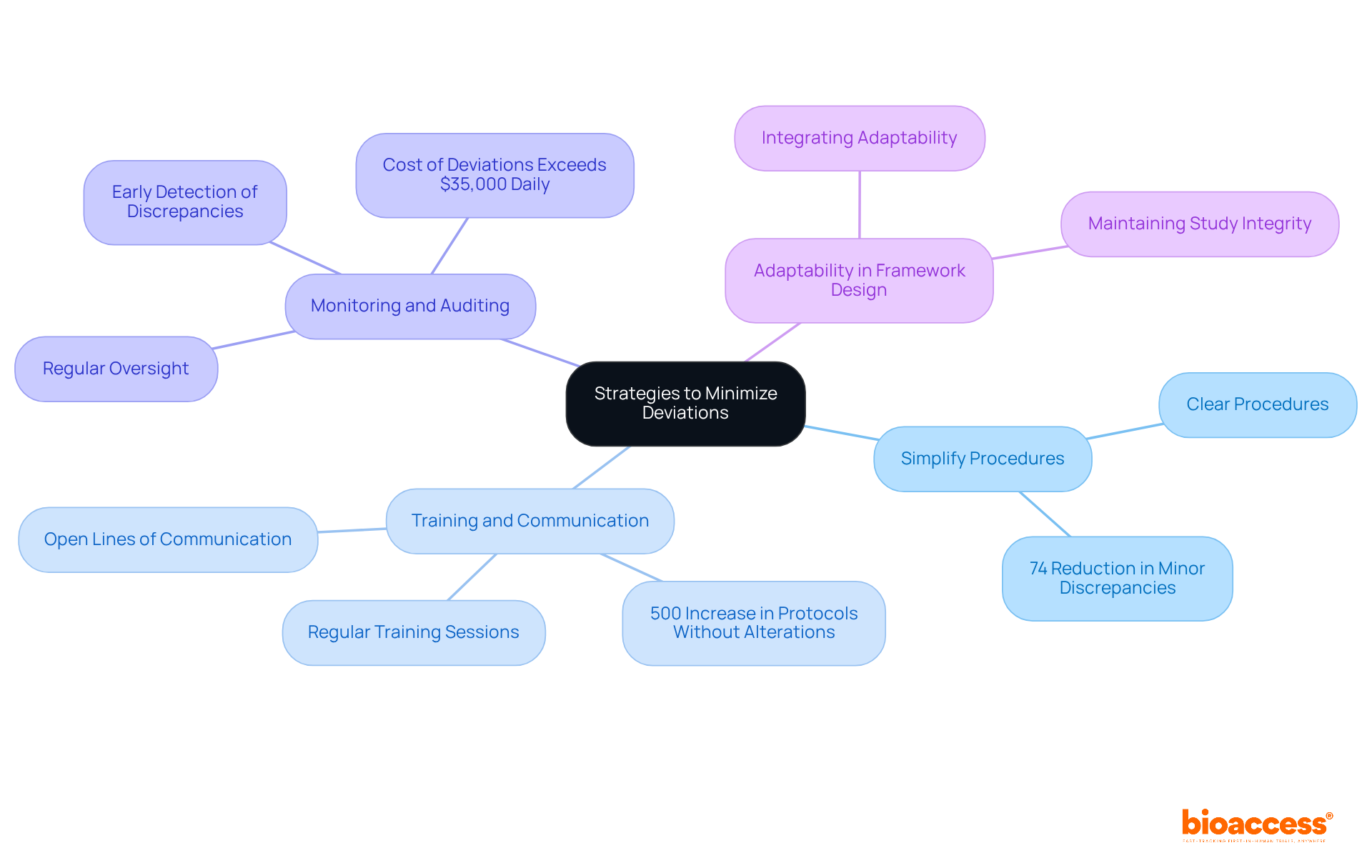
To minimize protocol deviations, researchers can implement several effective strategies:
Simplify Procedures: Creating clear procedures is essential. Simplified procedures decrease misunderstandings and improve adherence, as demonstrated by case studies indicating that streamlined designs result in higher compliance rates. For instance, procedures with Site Initiation Visits (SIVs) saw a 74% reduction in minor discrepancies, showcasing the effectiveness of simplification.
Training and Communication: Regular training sessions on guidelines are crucial. Research shows that focused investigator training greatly decreases noncompliance incidents, with a 500% rise in protocols without alterations after introducing Human Subjects Training. Maintaining open lines of communication allows staff to address questions or concerns promptly, fostering a culture of compliance.
Monitoring and Auditing: Conducting regular oversight of trial activities helps identify potential discrepancies early. Proactive auditing can reduce risks before they escalate, ensuring that procedures are adhered to as intended. The average expense of deviations can surpass $35,000 daily, making early detection essential.
Adaptability in Framework Design: Integrating adaptability into frameworks can accommodate unforeseen circumstances without compromising study integrity. This adaptability is essential for ensuring compliance, particularly in intricate experiments.
These strategies not only decrease the frequency of guideline breaches but also improve overall trial quality, ultimately aiding in managing protocol deviations in TGA-regulated trials. As Medha Datar points out, "Comprehending the nature and types of anomalies is essential for clinical operations teams to reduce risk and uphold data integrity.
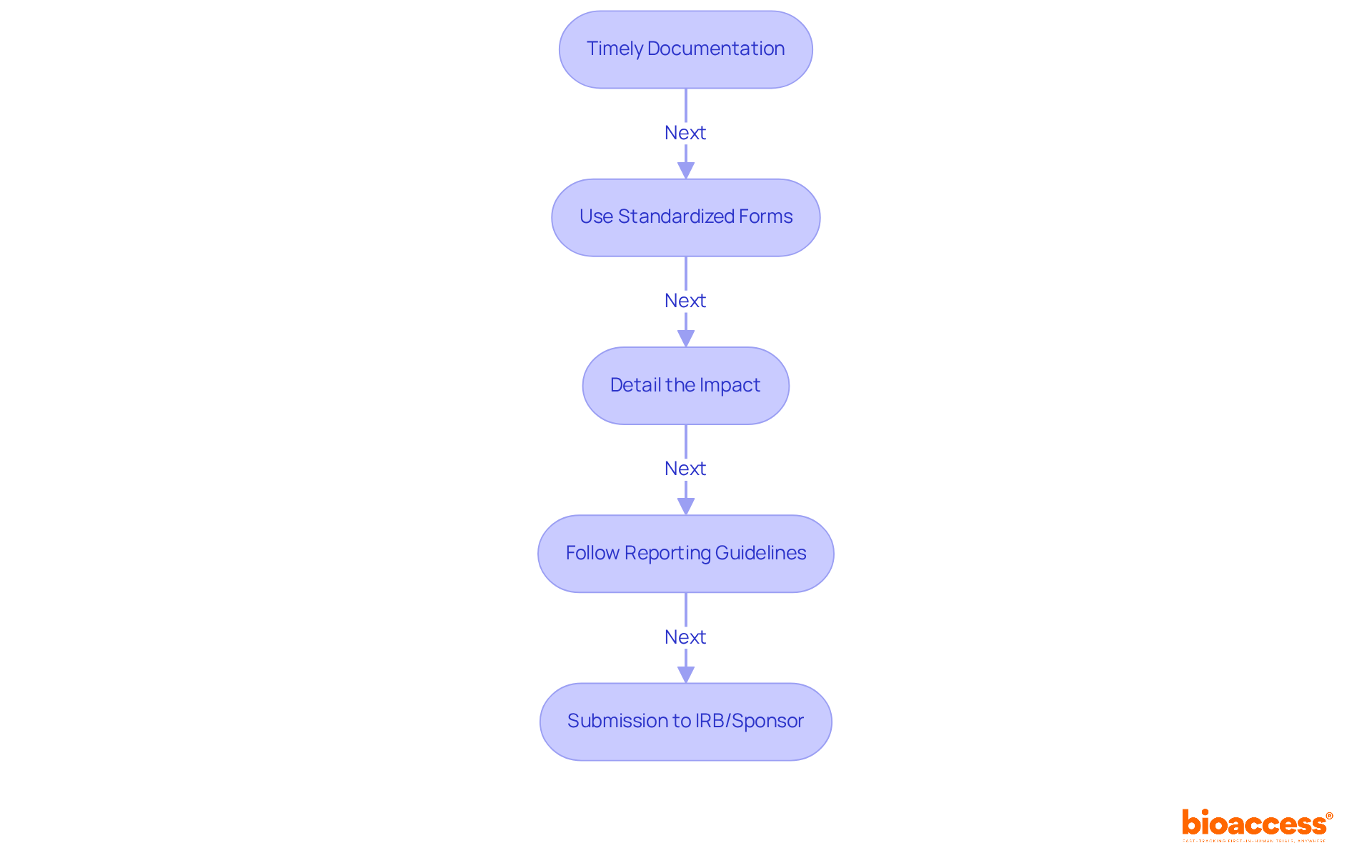
To effectively document and report protocol deviations, researchers must adhere to essential best practices:
Timely Documentation: Deviations should be recorded immediately upon identification, capturing all pertinent details to ensure accuracy. According to ICH E6(R2), documentation must be contemporaneous, objective, and precise. Delays can compromise compliance and data integrity. Notable discrepancies must be reported to the sponsor and IRB/IEC within 24-72 hours, underscoring the critical nature of timely documentation.
Use Standardized Forms: Implement standardized protocol exception forms to promote consistency in reporting across the study. This approach simplifies the documentation process and aligns with regulatory expectations, enhancing audit readiness. For instance, utilizing a Deviation Report Form can streamline the process and ensure all necessary information is captured.
Detail the Impact: Clearly articulate the variation's effect on participant safety and data integrity within the documentation. Evaluate whether the divergence impacts the integrity of study outcomes or poses dangers to participants, as substantial discrepancies must be reported without delay.
Follow Reporting Guidelines: Rigorously comply with the reporting guidelines set by the TGA, which detail specific timelines and necessary information for variances. Efficient handling of procedural variations is essential for managing protocol deviations in TGA-regulated trials and upholding regulations to ensure the integrity of clinical trials.
As clinical research experts emphasize, "Timeliness matters. For variations impacting primary endpoints or participant rights, submission to the IRB and sponsor must occur within 24-72 hours, based on local regulatory standards." By implementing these practices, researchers can enhance their compliance efforts, especially in managing protocol deviations in TGA-regulated trials, thereby safeguarding participant welfare and the credibility of study data.
Managing protocol deviations in TGA-regulated trials is crucial for upholding the integrity and reliability of clinical research. Understanding these deviations allows researchers to effectively tackle arising issues, ensuring participant safety and data quality remain intact. The necessity of adhering to regulatory standards is paramount, as it directly influences the validity of study outcomes.
Insights from the article reveal various types of protocol deviations - ranging from minor to serious - and outline strategies to minimize their occurrence. Implementing clear procedures, providing comprehensive training, and fostering open communication are essential steps that can significantly boost compliance. Moreover, timely documentation and strict adherence to reporting guidelines are vital in effectively managing these deviations, ensuring regulatory requirements are met and the trial's integrity is preserved.
In summary, mastering protocol deviation management is of utmost importance. As clinical trials evolve, so must the strategies to navigate compliance complexities. By prioritizing best practices in managing protocol deviations, researchers can advance clinical research while safeguarding participant welfare and ensuring the credibility of their findings. Taking proactive measures today will lay the groundwork for successful trials in the future.
What are protocol deviations in clinical research?
Protocol deviations are any alterations or deviations from the study design or procedures outlined in the approved clinical research document. They can occur unintentionally or deliberately and can significantly impact the integrity of the trial.
Why is it important to understand the implications of protocol deviations?
Understanding the implications of protocol deviations is crucial as they can affect data quality, participant safety, and the overall validity of study results. Even minor variations can lead to serious issues in data interpretation and regulatory compliance if they occur repeatedly.
What types of protocol deviations exist?
Protocol deviations can be classified into four categories: 1. Minor Deviations - Do not significantly impact participant safety or data integrity. 2. Major Deviations - Can have serious consequences for participant safety or data integrity. 3. Serious Deviations - Significant breaches that require immediate reporting to regulatory bodies. 4. Intentional Deviations - Planned changes made with prior approval, documented in protocol amendments.
What are the consequences of minor and major deviations?
Minor deviations typically do not pose immediate risks, while major deviations can compromise the validity of study results and potentially endanger participant well-being. Serious deviations can lead to regulatory penalties and jeopardize the trial's integrity.
How can protocol deviations be managed in clinical trials?
Managing protocol deviations is essential for identifying and maintaining the reliability of clinical research. This includes thorough training and compliance with guidelines to reduce inconsistencies and improve the overall quality of clinical trials.
What services does bioaccess® provide related to clinical trial management?
Bioaccess® offers comprehensive clinical trial management services, including feasibility studies, site selection, compliance reviews, and project management, ensuring that protocol issues are effectively addressed.