


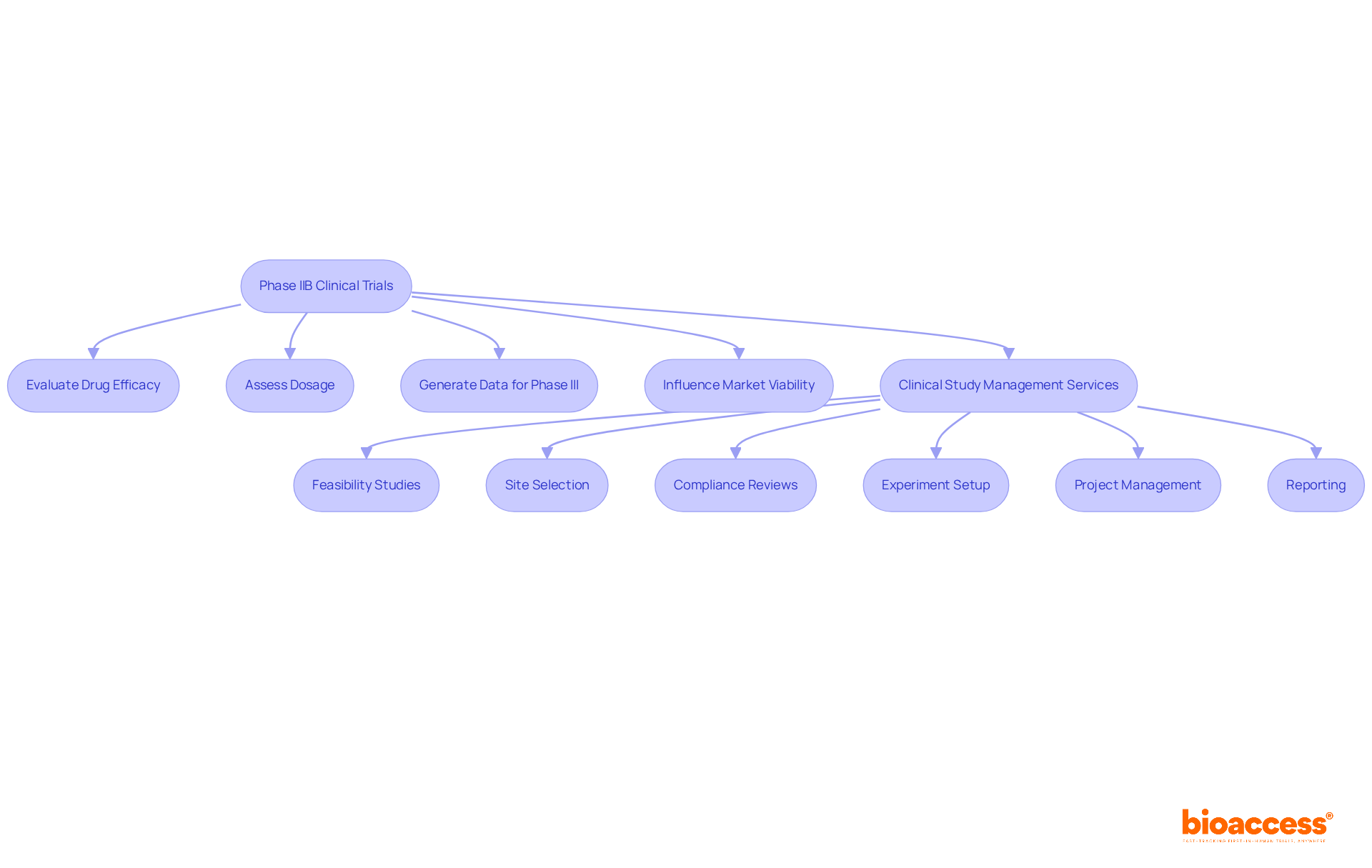
Phase IIB clinical trials play a pivotal role in assessing a drug's effectiveness and fine-tuning dosing strategies, acting as a crucial bridge to the later stages of drug development. These trials are not without their challenges; they often encounter significant regulatory hurdles and recruitment issues. However, specialized management services, such as those offered by bioaccess, can effectively address these obstacles, thereby increasing the chances of successful outcomes.
In the ever-evolving Medtech landscape, the importance of collaboration cannot be overstated. Bioaccess stands out by providing tailored solutions that tackle the key challenges faced during Phase IIB trials. By leveraging their expertise, clinical researchers can navigate the complexities of trial management more efficiently, ensuring that their studies remain on track and within regulatory guidelines.
Ultimately, the success of Phase IIB clinical trials hinges on effective partnerships. Engaging with specialized management services like bioaccess not only enhances the likelihood of achieving favorable results but also fosters a more streamlined approach to drug development. As the industry continues to grow, now is the time to consider how collaboration can elevate your clinical research efforts.
Phase IIB clinical trials represent a pivotal moment in the drug development process, marking the shift from safety assessments to a thorough evaluation of therapeutic efficacy. These trials are crucial, as they not only determine optimal dosing but also produce vital data that can influence a drug's market trajectory. Yet, the complexities of this phase pose significant challenges, including regulatory hurdles and recruitment difficulties.
What strategies can researchers implement to navigate these obstacles and secure successful outcomes in Phase IIB trials?
Phase IIB clinical trials represent a pivotal phase in the drug development continuum, primarily aimed at evaluating the effectiveness of a treatment across a larger patient population. Typically involving several hundred participants, these studies focus on assessing the therapeutic impact of the drug at the proposed dosage. Unlike Stage I studies, which prioritize safety, phase IIB clinical trials are designed to optimize dosing schedules and confirm the drug's efficacy. This stage is essential, as it generates the crucial data needed to advance to Stage III studies, where the treatment is evaluated in an even broader population.
The outcomes of phase IIB clinical trials can significantly influence a medication's trajectory, determining its market viability and potential to enhance patient care. For instance, the second stage study of GH001 for treatment-resistant depression demonstrated substantial effectiveness, leading to a shift in treatment protocols. Moreover, statistics reveal that only 29% of drug programs achieve proof of concept at this stage, highlighting the hurdles encountered in moving from phase I clinical trials to phase IIB clinical trials and then to Phase III.
To navigate these challenges, comprehensive clinical study management services, such as those offered by bioaccess, are indispensable. They facilitate:
These services ensure that trials are executed efficiently and effectively. Notably, bioaccess's ability to enroll treatment-naive groups 50% faster than Western locations, coupled with potential savings of $25K per patient through FDA-ready data, significantly enhances the feasibility of progressing to subsequent study phases.
Expert insights underscore that the success of phase IIB clinical trials is vital for informed decision-making regarding further development, establishing this phase as a cornerstone of effective drug development strategies. Collaboration with specialized services like bioaccess is essential for overcoming the complexities of clinical research and ensuring successful outcomes.
Phase IIB clinical trials play a pivotal role in drug development, as they are primarily aimed at evaluating a drug's effectiveness, determining optimal dosing, and identifying potential side effects. These trials employ robust methodologies to ensure reliable results, including:
Recent statistics indicate that approximately 33% of medications in phase IIB clinical trials progress to the next stage, underscoring the importance of these trials in the drug development process. For example, the NRG-HN002 study, which investigates de-intensification therapies for oropharyngeal cancer, exemplifies the application of a controlled randomized design to assess treatment efficacy while monitoring adverse effects.
By utilizing these methodologies, phase IIB clinical trials provide critical insights into a drug's therapeutic potential, which guide further development and regulatory submissions. With bioaccess®, these studies can be expedited, achieving patient enrollment 50% faster than traditional methods, resulting in significant savings of $25K per patient. Our comprehensive clinical study management services include feasibility assessments, site selection, compliance evaluations, study setup, import permits, project oversight, and reporting, ensuring a streamlined process that meets regulatory standards.

Phase IIB studies encounter a range of regulatory obstacles that can complicate the research process. Understanding these challenges is crucial for clinical research success.
Regulatory Approval is a primary hurdle. Securing approval from regulatory bodies like the FDA or EMA is essential before trials can commence. This process can be lengthy, often requiring extensive documentation and strict adherence to specific guidelines. bioaccess® specializes in streamlining this process, enabling startups to navigate regulatory landscapes efficiently and advance to the next phase of their studies more swiftly.
Another significant issue is Compliance with Good Clinical Practice (GCP). Adhering to GCP guidelines is not just a regulatory requirement; it’s vital for maintaining the integrity of the study and ensuring participant safety. Non-compliance can lead to severe penalties, including delays and potential disqualification of data. In 2025, compliance rates in phase IIB clinical trials showed variability, with some research indicating adherence issues that could jeopardize the outcomes of these trials. For example, one-third of studies enrolled only 5% of the planned participants, underscoring recruitment challenges that affect compliance. bioaccess® provides comprehensive support in site feasibility and investigator selection, helping to mitigate these recruitment issues.
Information Management and Reporting are also critical. Accurate data collection and reporting are essential for regulatory submissions. Inconsistencies can trigger audits and raise concerns about the validity of the study. A notable case in 2025 highlighted compliance issues where data inconsistencies led to a hearing's suspension, emphasizing the need for meticulous information management. Additionally, the challenges of patient recruitment in pivotal studies can significantly impact adherence and overall success. bioaccess® offers project management and monitoring services to ensure data integrity throughout the testing process.
Addressing these challenges requires a proactive approach, involving consistent communication with regulatory bodies and thorough planning to ensure compliance. As industry expert Patricio Ledesma points out, maintaining strict GCP standards is essential for the success of phase IIB clinical trials and for ensuring the overall integrity of clinical research. Collaborating with agile Contract Research Organizations (CROs) like bioaccess® can provide invaluable support in effectively managing compliance issues. Furthermore, incorporating ethical principles from the Belmont Report, such as respect for individuals and beneficence, is crucial for ensuring participant safety and adherence to ethical standards in clinical studies.

Effective recruitment and data management strategies are crucial for the success of phase IIB clinical trials. Here are key best practices to consider:
Targeted Recruitment: Focus on identifying and engaging specific patient populations that align with the trial's inclusion criteria. Leverage patient registries, social media platforms, and healthcare providers to effectively reach potential participants. This focused approach can significantly enhance enrollment rates. Notably, collaborations such as that of GlobalCare Clinical Studies with bioaccess™ have demonstrated the effectiveness of utilizing local expertise, reducing clinical study subject recruitment time by over 50%.
Clear Communication: Ensure that potential participants receive clear and concise information regarding the study's purpose, procedures, and potential risks and benefits. Transparency fosters trust and increases the likelihood of participation, as individuals feel more informed about their involvement. As Ken Getz noted, "Trials are intended to generate data on how a drug works, and you can’t generate that data if you don’t have participants in the study."
Retention Strategies: Develop effective strategies to sustain participant involvement during the study. Regular follow-ups, timely reminders, and providing support throughout the trial process are crucial for minimizing dropout rates and ensuring participant satisfaction. Building trust with communities is vital, as highlighted in various studies where participants noted that establishing relationships with community leaders helps build trust. The collaboration between GlobalCare and bioaccess™ also achieved an impressive retention rate of over 95%, showcasing the impact of effective engagement strategies.
Information Management Systems: Implement electronic information capture (EIC) systems to streamline information collection processes and enhance precision. Regular monitoring of information for completeness and consistency is essential to maintain high-quality standards and ensure compliance with regulatory requirements. The incorporation of sophisticated information management solutions can greatly enhance the effectiveness of testing procedures.
By prioritizing these strategies, researchers can improve recruitment efforts and optimize data management processes, ultimately contributing to the success of phase IIB clinical trials.

Phase IIB clinical trials represent a pivotal moment in the drug development process, concentrating on the effectiveness and optimal dosing of treatments across broader patient populations. Transitioning from safety assessments to evaluating therapeutic impact, these trials are crucial in determining whether a drug can advance to subsequent testing stages. The importance of this phase is immense, as it establishes the foundation for successful regulatory submissions and, ultimately, market approval.
In this article, we've explored several key insights, including the methodologies utilized in Phase IIB trials, such as randomized controlled trials and adaptive designs, which significantly enhance the reliability of outcomes. The role of robust clinical study management services, like those offered by bioaccess, has also been emphasized, demonstrating how they can streamline processes, mitigate regulatory challenges, and improve participant recruitment and retention. Collectively, these factors contribute to the success of Phase IIB trials, with only about one-third of medications progressing to the next stage, highlighting the necessity for strategic planning and execution.
In summary, the success of Phase IIB clinical trials relies on a thorough understanding of their objectives, challenges, and best practices. As the drug development landscape grows increasingly competitive, leveraging specialized services and innovative methodologies becomes essential for overcoming obstacles and enhancing the likelihood of successful outcomes. Stakeholders in the clinical research field are urged to adopt these strategies and collaborate with expert organizations to ensure that the potential of new therapies is fully realized, ultimately benefiting patient care and advancing medical science.
What are Phase IIB clinical trials?
Phase IIB clinical trials are a crucial phase in drug development focused on evaluating the effectiveness of a treatment across a larger patient population, typically involving several hundred participants.
What is the primary goal of Phase IIB clinical trials?
The primary goal of Phase IIB clinical trials is to assess the therapeutic impact of a drug at the proposed dosage, optimizing dosing schedules, and confirming the drug's efficacy.
How do Phase IIB trials differ from Phase I trials?
Unlike Phase I trials, which prioritize safety, Phase IIB trials are designed to evaluate the drug's effectiveness and optimize its dosing.
Why are Phase IIB clinical trials important?
Phase IIB clinical trials generate crucial data needed to advance to Phase III studies and can significantly influence a medication's market viability and potential to enhance patient care.
Can you provide an example of a Phase IIB clinical trial's impact?
The Phase IIB study of GH001 for treatment-resistant depression demonstrated substantial effectiveness, leading to a shift in treatment protocols.
What percentage of drug programs achieve proof of concept in Phase IIB trials?
Only 29% of drug programs achieve proof of concept at the Phase IIB stage.
What services do clinical study management companies like bioaccess provide?
They offer services such as feasibility studies, site selection, compliance reviews, experiment setup, project management, and reporting to ensure efficient trial execution.
How does bioaccess enhance the feasibility of progressing to subsequent study phases?
Bioaccess can enroll treatment-naive groups 50% faster than Western locations and potentially save $25K per patient through FDA-ready data.
Why is collaboration with specialized services important in Phase IIB trials?
Collaboration with specialized services is essential for overcoming the complexities of clinical research and ensuring successful outcomes in drug development.