


Efficient management of protocol deviations stands as a cornerstone for upholding integrity in clinical trials, especially under the stringent Halmed regulations established by the Croatian Agency for Medicinal Products and Medical Devices. This guide provides essential insights into navigating the complexities of protocol deviations, detailing critical practices for identification, documentation, and reporting that ensure compliance and prioritize participant safety. As the landscape of clinical research continues to evolve, researchers must ask: how can they effectively adapt their strategies to minimize discrepancies and enhance study outcomes?
Efficient oversight of protocol deviation handling under Halmed regulations in research studies is crucial for maintaining the integrity of clinical investigations. A comprehensive understanding of Halmed regulations, established by the Croatian Agency for Medicinal Products and Medical Devices, is essential. These regulations delineate specific requirements for identifying, documenting, and reporting discrepancies, which are vital for compliance and participant safety, particularly in the context of protocol deviation handling under Halmed regulations.
Definition of Protocol Deviations: According to Halmed regulations, a protocol deviation is any unapproved departure from the approved clinical trial protocol.
Categories of Deviations: Deviations fall into minor and major categories, each carrying distinct implications for study integrity and participant safety. Minor variations may have minimal impact, while major discrepancies can significantly compromise data quality and participant rights.
Regulatory Compliance: Halmed enforces stringent compliance standards, including specific deadlines for reporting inconsistencies and the necessary documentation to ensure clarity and accountability in medical studies. Recent updates underscore the importance of timely reporting for protocol deviation handling under Halmed regulations to uphold compliance and safeguard participant safety.
Resources: Staying informed about regulatory changes is paramount. Utilizing Halmed's official guidelines and resources provides critical insights into compliance necessities and effective methods for protocol deviation handling under Halmed regulations.
Understanding these essential components empowers clinical research experts to navigate the complexities of study variations effectively. Proactive management of protocol deviation handling under Halmed regulations not only enhances study outcomes but also prioritizes participant safety. For instance, a recent study highlighted the need for improved support and education for investigative sites, which can significantly reduce inconsistencies and enhance study performance. Furthermore, insights from industry specialists indicate a strong correlation between system complexity and the likelihood of inconsistencies, emphasizing the importance of clear and manageable study designs. By integrating these insights into your research management strategies, you can foster a culture of compliance and excellence in clinical studies.

Recognizing inconsistencies in procedures is crucial for maintaining the integrity of clinical research. A detailed method and thorough comprehension of research guidelines are essential for effective protocol deviation handling under halmed regulations. To achieve this, consider the following best practices:
By systematically recognizing discrepancies, you can address problems swiftly, thus preserving the integrity of your research study and minimizing the risk of costly procedural changes, which can exceed $35,000 per day. Furthermore, achieving zero significant guideline discrepancies should be a key success metric for site training, as emphasized by an experienced research audience during a 2021 webinar.
Accurate recording of protocol variations is essential for ensuring compliance, particularly in the context of protocol deviation handling under Halmed regulations, and maintaining data integrity in clinical studies. To enhance your documentation process, consider implementing the following best practices:
By adhering to these documentation methods, you can ensure that all procedure variations are accurately recorded, thereby supporting protocol deviation handling under Halmed regulations and enhancing the overall integrity of your research studies. Additionally, consider utilizing tools like Iodine's AwareCDI, which can bolster Clinical Documentation Improvement (CDI) programs by identifying gaps in documentation.
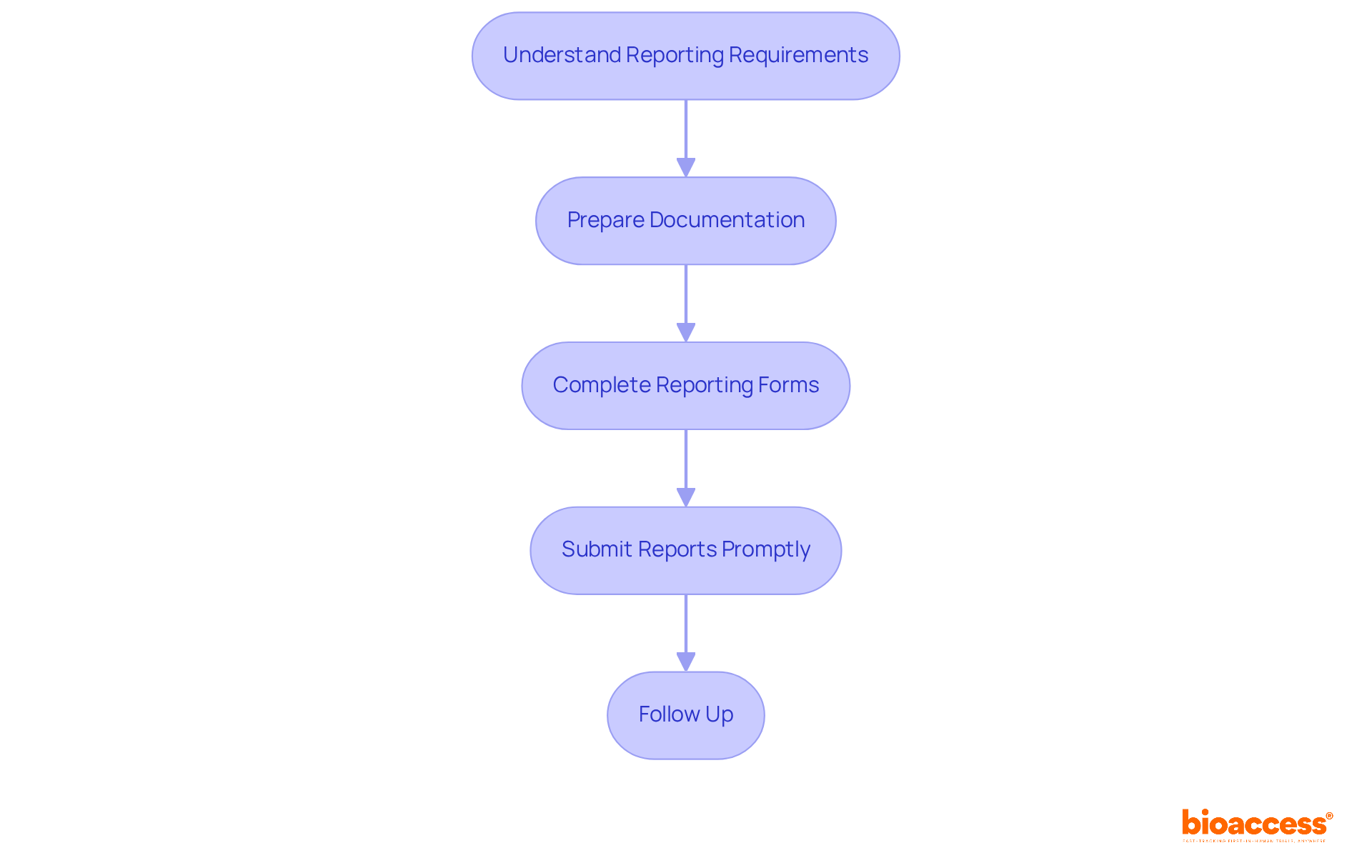
It is essential for maintaining compliance and ensuring the integrity of research studies to report protocol deviation handling under Halmed regulations. At bioaccess, we offer comprehensive clinical study management services, including feasibility assessments and site selection, guiding you through every step of this process. Follow these steps for effective reporting:
Understand Reporting Requirements: Familiarize yourself with Halmed's specific reporting guidelines, including the types of irregularities that must be reported and the associated timelines. Significant discrepancies impacting participant safety or trial integrity must be reported without delay, while minor discrepancies should be documented and assessed during ongoing applications. Unreported discrepancies can lead to inconsistencies that may compromise the study’s outcomes.
Prepare Documentation: Gather all essential paperwork related to the discrepancy, including a detailed discrepancy log, impact assessment, corrective actions taken, and root cause analysis. Documentation must be contemporaneous, objective, and precise to meet regulatory standards. Our team at bioaccess can assist in reviewing and providing feedback on study documents to ensure compliance with country requirements.
Complete Reporting Forms: Accurately fill out the required reporting forms, ensuring that all information is thorough and reflects the details of the variation. This includes the date of occurrence, a detailed description, assessed impact, and preventive steps taken.
Submit Reports Promptly: Submit reports to Halmed within the specified timeframe. Prompt reporting is crucial; for FDA-regulated studies, significant discrepancies impacting participant safety or study validity must be communicated within 5 to 15 days of identification. This demonstrates compliance and transparency, helping to avoid potential regulatory audits. Our project management services ensure that all timelines are met efficiently.
Follow Up: After submission, confirm receipt of the report with Halmed and address any questions or requests for additional information. This proactive approach helps maintain open communication and ensures that all necessary details are provided. At bioaccess, we prioritize effective communication and support throughout the study process.
By following these reporting methods and leveraging bioaccess's expertise in regulatory matters for medical devices and in vitro diagnostics, you can effectively convey all variations to Halmed, thus facilitating protocol deviation handling under Halmed regulations and enhancing the overall integrity of your research study.
Effective protocol deviation handling under halmed regulations is crucial for reducing the effect of protocol variances and maintaining the integrity of research studies. To effectively address deviations, consider implementing the following strategies, leveraging the comprehensive clinical trial management services offered by bioaccess:
By proactively mitigating the impact of protocol deviations through protocol deviation handling under halmed regulations, you can enhance the reliability of your clinical trial outcomes and ensure participant safety, all supported by the comprehensive services of bioaccess.
Efficient management of protocol deviations under Halmed regulations is crucial for maintaining the integrity and safety of clinical trials. Understanding these regulations allows researchers to navigate the complexities of protocol deviations effectively, ensuring compliance and safeguarding participant rights. This expertise not only elevates the quality of clinical investigations but also cultivates a culture of accountability and excellence within research teams.
Key insights from the article underscore the necessity of recognizing, documenting, and reporting protocol deviations promptly. By emphasizing best practices such as:
Researchers can significantly reduce the risks associated with protocol deviations. Furthermore, proactive communication with stakeholders and regular audits can bolster compliance and enhance the overall integrity of clinical studies.
The importance of adhering to Halmed regulations cannot be overstated. Implementing the strategies outlined in this guide enables clinical researchers to improve study outcomes while contributing to the broader goal of advancing medical science with a focus on participant safety. By embracing these practices, researchers will achieve more reliable findings and establish a stronger foundation for future clinical trials.
What are Halmed regulations regarding protocol deviations?
Halmed regulations, established by the Croatian Agency for Medicinal Products and Medical Devices, outline specific requirements for identifying, documenting, and reporting protocol deviations in clinical trials to maintain study integrity and participant safety.
How is a protocol deviation defined under Halmed regulations?
A protocol deviation is defined as any unapproved departure from the approved clinical trial protocol.
What are the categories of protocol deviations?
Protocol deviations are categorized into minor and major deviations. Minor deviations may have minimal impact, while major deviations can significantly compromise data quality and participant rights.
What are the compliance standards enforced by Halmed?
Halmed enforces stringent compliance standards, including specific deadlines for reporting deviations and requirements for documentation to ensure clarity and accountability in medical studies.
Why is timely reporting important in protocol deviation handling?
Timely reporting is crucial for compliance with Halmed regulations and for safeguarding participant safety.
How can researchers stay informed about regulatory changes?
Researchers can utilize Halmed's official guidelines and resources to gain insights into compliance necessities and effective methods for handling protocol deviations.
What best practices should be followed to identify protocol deviations in clinical trials?
Best practices include reviewing clinical study guidelines, supervising experiment activities, conducting regular audits, training staff, and documenting observations.
Why is staff training important in identifying protocol deviations?
Staff training ensures that team members are prepared to identify discrepancies quickly, reducing the frequency and seriousness of deviations.
What role does documentation play in protocol deviation handling?
Proper documentation of observations helps maintain transparency, ensures compliance, and supports audit readiness, trend analysis, and regulatory credibility.
What is a key success metric for site training regarding protocol deviations?
Achieving zero significant guideline discrepancies is emphasized as a key success metric for site training.