Introduction
Navigating the complexities of clinical trial applications in Montenegro presents significant challenges for researchers and pharmaceutical companies. A well-structured drug dossier is not merely a formality; it is a critical component that can determine the success of a submission. This guide explores the essential elements required for a successful Clinical Trial Application (CTA) in Montenegro, providing insights into the necessary documentation and regulatory compliance to enhance approval chances. With evolving regulations and stringent requirements, how can you ensure that your application stands out in this competitive landscape?
Understand Montenegro's Clinical Trial Application Requirements
To effectively navigate Montenegro's Clinical Trial Application (CTA) system, understanding the key components mandated by the Law on Medicines and Medical Devices is essential. This knowledge not only simplifies the application process but also significantly enhances the chances of approval. The critical elements of a successful CTA include:
- Application Form: Accurately complete the official CTA form, ensuring all sections are thoroughly filled out.
- Study Protocol: Submit a comprehensive study protocol detailing the objectives, methodology, and statistical analysis plan, which serves as the backbone of your research.
- Investigator's Brochure: Provide a detailed brochure that includes essential information about the investigational product, encompassing safety and efficacy data.
- Informed Consent Forms: Develop clear and concise informed consent forms that effectively communicate the study's purpose and associated risks to participants.
- Ethics Committee Approval: Obtain consent from a recognized ethics committee, a necessary step before presenting, ensuring adherence to ethical standards.
By familiarizing yourself with these requirements, you can enhance the thoroughness and adherence of your application, significantly lowering the risk of delays or rejections in the approval procedure.

Identify Essential Documentation for Drug Dossier
Successful drug dossier structure for Montenegro CTA submission relies on the inclusion of several essential documents. This checklist serves as a vital guide to streamline your submission process:
- Cover Letter: A formal letter outlining the submission's purpose and details about the investigational product.
- Completed Application Form: Ensure the CTA form is filled out completely and accurately to avoid delays.
- Study Protocol: A detailed document describing the study design, objectives, and methodology, crucial for regulatory review.
- Investigator's Brochure: This document must contain all pertinent information about the investigational product, including preclinical and clinical data, to inform all stakeholders.
- Informed Consent Forms: Tailored to the specific study, these forms must receive approval from the ethics committee to ensure ethical compliance.
- Safety Data: Include any available safety data from prior studies or trials, as this information is vital for assessing risk.
- Manufacturing Information: Provide comprehensive details about the production methods and quality control measures to demonstrate compliance with regulatory standards.
- Language Compliance: All documents must be prepared in either Montenegrin or English to comply with local regulations.
- Proof of Marketing Authorization: Ensure to include proof of valid marketing authorization for the reference medicinal product in the specified country.
- Agreement from Marketing Authorization Holder: Proof of agreement from the marketing authorization holder of the reference product is required for the application.
By meticulously compiling the drug dossier structure for Montenegro CTA submission, you will not only streamline the application process but also significantly enhance the likelihood of obtaining approval. Statistics indicate that submissions following this organized method achieve a greater success rate, underscoring the significance of comprehensive preparation in navigating the regulatory landscape of the region.

Ensure Compliance with Local Regulatory Guidelines
To ensure compliance with Montenegro's regulatory guidelines, it's essential to adopt effective strategies that enhance your clinical trial's credibility and success rate.
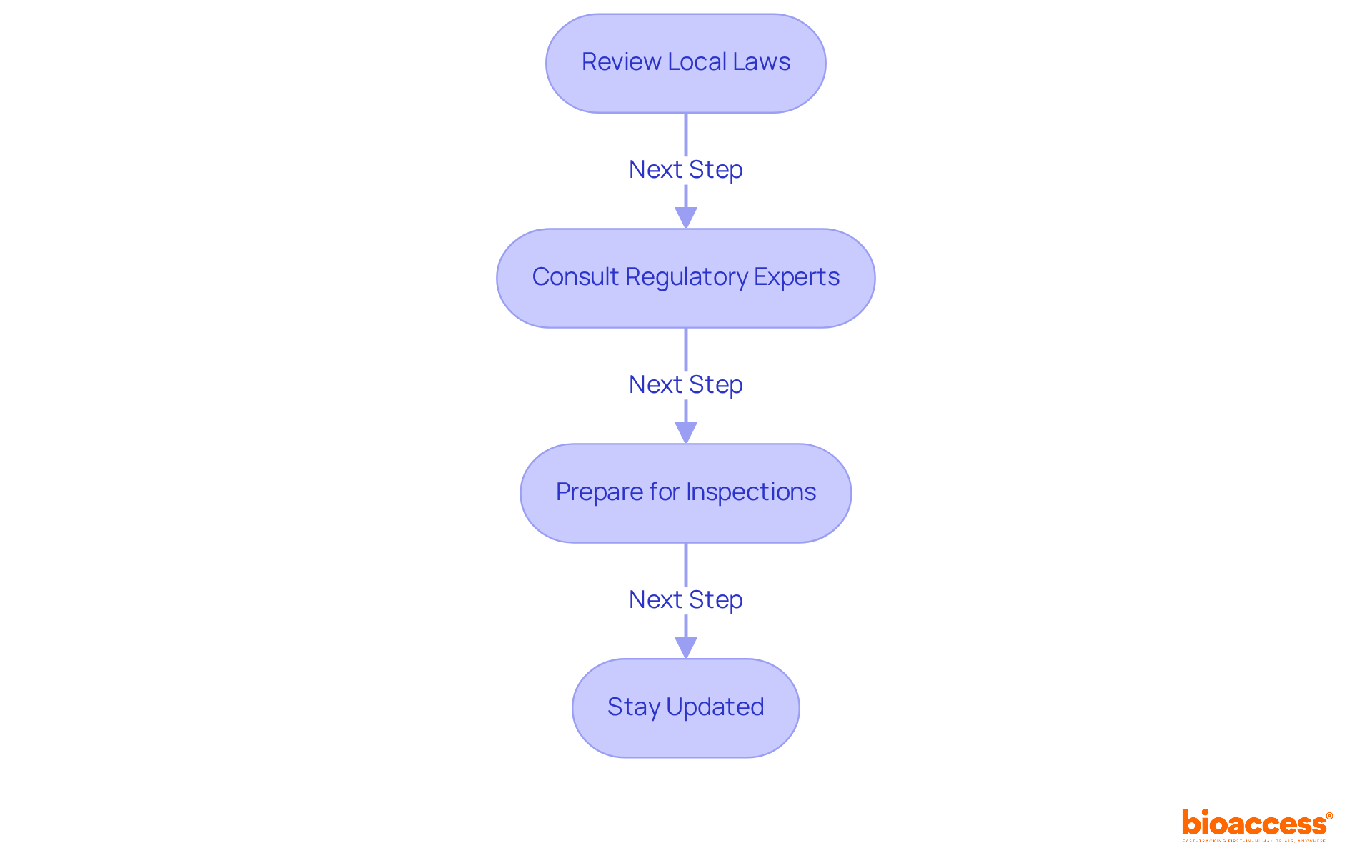
- Review Local Laws: Start by gaining a comprehensive understanding of the Law on Medicines and Medical Devices, along with the guidelines from the Agency for Medicines and Medical Devices of Montenegro. This foundational knowledge is crucial for navigating the regulatory landscape effectively.
- Consult Regulatory Experts: Collaborate with local regulatory consultants who possess in-depth knowledge of Montenegro's clinical trial regulations. Their insights can clarify complex requirements and simplify the application procedure, making your path smoother.
- Prepare for Inspections: Anticipate potential inspections by regulatory authorities. Ensure that all documentation is meticulously organized and readily accessible; this preparation will facilitate a smoother review process.
- Stay Updated: Regularly monitor updates to regulations or guidelines that may affect your entry. The regulatory environment in the region is evolving, and staying informed will help you adapt to any changes promptly.
By prioritizing these compliance strategies, you not only enhance the credibility of your clinical trial but also significantly improve the likelihood of a successful submission.
Obtain Ethical Approvals and Committees' Endorsements
To secure ethical approvals and endorsements from committees in Montenegro, follow these essential steps:
- Identify the Relevant Ethics Committee: Determine the appropriate ethics committee based on your study's scope and location. This is crucial for compliance and integrity.
- Prepare Required Documents: Compile all essential documents, including the study protocol, informed consent forms, Investigator's Brochure, and any additional supporting materials. Thorough documentation is vital for confirming operational compliance and facilitating a seamless review. Insufficient documentation can lead to considerable delays in the review process, with hold-ups varying from 97 to 396 days.
- Submit Application: Submit your application to the ethics committee, ensuring that all documents are complete and formatted according to their specific guidelines. Clarity in your work can significantly reduce the likelihood of requests for clarification.
- Respond to Feedback: Be prepared to address any questions or concerns raised by the committee during their review. Timely follow-ups can facilitate prompt reviews and demonstrate your dedication to moral standards.
Once you receive approval, ensure you obtain formal documentation of the moral approval, as this is necessary for the drug dossier structure for Montenegro CTA submission. The typical length of the review for drug trials in Montenegro, which is important for the drug dossier structure for Montenegro CTA submission, usually ranges from 1 to 3 months.
Obtaining necessary approvals not only meets legal requirements but also underscores your commitment to participant safety and principled research practices. The integrity review process, overseen by integrity committees, plays a crucial role in promoting medical research while protecting the rights and welfare of participants. As emphasized, "The significance of ethical reviews in drug trials cannot be overstated." Additionally, understanding the Law on Medicines and Medical Devices is essential for navigating the regulatory landscape in Montenegro.

Conclusão
Mastering the drug dossier structure for a Clinical Trial Application (CTA) in Montenegro is crucial for ensuring a successful submission. Understanding local requirements, documentation, and ethical considerations streamlines the application process and significantly boosts the chances of obtaining necessary approvals. By following the outlined guidelines, researchers can confidently navigate the complexities of Montenegro's regulatory landscape.
Key elements include:
- The importance of accurately completing the application form
- Providing a detailed study protocol
- Compiling essential documents such as the Investigator's Brochure and informed consent forms
- Securing ethical approvals
- Grasping local regulations
Each of these aspects is vital in creating a well-structured drug dossier that meets regulatory authorities' expectations.
Given these insights, stakeholders in the pharmaceutical and clinical research industries must prioritize meticulous preparation and compliance with Montenegro's regulatory guidelines. Embracing these practices not only enhances the integrity of clinical trials but also fosters a culture of ethical research that ultimately benefits participants and the broader scientific community. By proactively mastering the drug dossier structure, researchers can pave the way for successful clinical trials in Montenegro and contribute to advancements in medical research.
Frequently Asked Questions
What is the purpose of understanding Montenegro's Clinical Trial Application (CTA) requirements?
Understanding Montenegro's CTA requirements simplifies the application process and enhances the chances of approval.
What is the first critical element of a successful CTA?
The first critical element is the Application Form, which must be accurately completed with all sections thoroughly filled out.
What should be included in the Study Protocol?
The Study Protocol should detail the objectives, methodology, and statistical analysis plan, serving as the backbone of the research.
What information is required in the Investigator's Brochure?
The Investigator's Brochure must include essential information about the investigational product, including safety and efficacy data.
Why are Informed Consent Forms important in the CTA process?
Informed Consent Forms are important because they communicate the study's purpose and associated risks to participants clearly and concisely.
What is the role of the Ethics Committee in the CTA process?
The Ethics Committee must provide approval, ensuring adherence to ethical standards before the application can be presented.
How can familiarizing oneself with these requirements benefit the application process?
Familiarizing oneself with these requirements enhances the thoroughness and adherence of the application, significantly lowering the risk of delays or rejections in the approval procedure.
List of Sources
- Understand Montenegro's Clinical Trial Application Requirements
- Master Gene Therapy Trial Regulation in Montenegro: A Complete Guide (https://bioaccessla.com/blog/master-gene-therapy-trial-regulation-in-montenegro-a-complete-guide)
- Clinical trials - Cinmed (https://cinmed.me/en/humane-medicines/clinical-trials)
- Regulatory Submission Checklist for Montenegro Drug Trials (https://bioaccessla.com/blog/regulatory-submission-checklist-for-montenegro-drug-trials)
- Identify Essential Documentation for Drug Dossier
- Regulatory Submission Checklist for Montenegro Drug Trials (https://bioaccessla.com/blog/regulatory-submission-checklist-for-montenegro-drug-trials)
- Montenegro Drug Product Registration | OMC Medical Limited (https://omcmedical.com/montenegro-drug-product-registration)
- Ensure Compliance with Local Regulatory Guidelines
- Montenegro Amends Legislation on Restricted Chemicals and Persistent Organic Pollutants (https://sgs.com/en-ng/news/2025/10/safeguards-15325-montenegro-amends-legislation-on-restricted-chemicals-and-persistent-organic-pollutants)
- Compliance requirements for a Montenegro company - Business Registration & Company Formation in Montenegro (https://companyformationmontenegro.me/blog/2019/11/08/compliance-requirements-for-a-montenegro-company)
- Master Gene Therapy Trial Regulation in Montenegro: A Complete Guide (https://bioaccessla.com/blog/master-gene-therapy-trial-regulation-in-montenegro-a-complete-guide)
- Placing a medicine on the market - Cinmed (https://cinmed.me/en/humane-medicines/placing-a-medicine-on-the-market)
- Regulatory Submission Checklist for Montenegro Drug Trials (https://bioaccessla.com/blog/regulatory-submission-checklist-for-montenegro-drug-trials)
- Obtain Ethical Approvals and Committees' Endorsements
- Medical Writing for Device Trials: MDR/IVDR⚕️ Pure Clinical (https://pureclinical.eu/clinical-trials/medical-writing)
- Regulatory Submission Checklist for Montenegro Drug Trials (https://bioaccessla.com/blog/regulatory-submission-checklist-for-montenegro-drug-trials)
- Master the Ethical Review Process for Drug Trials in Montenegro (https://bioaccessla.com/blog/master-the-ethical-review-process-for-drug-trials-in-montenegro)
- Global comparison of research ethical review protocols: insights from an international research collaborative - PMC (https://pmc.ncbi.nlm.nih.gov/articles/PMC11975204)






