


Navigating the pediatric trial authorization process in Bulgaria presents a unique set of challenges and opportunities for researchers eager to advance child health. Pediatric studies make up a significant portion of clinical research, making it essential to grasp the regulatory landscape - especially the recent changes mandated by the European Union.
How can stakeholders ensure compliance and streamline their submissions in a rapidly evolving system? This guide explores the critical steps, required documentation, and compliance measures necessary to master the pediatric trial authorization process in Bulgaria, paving the way for successful clinical outcomes.
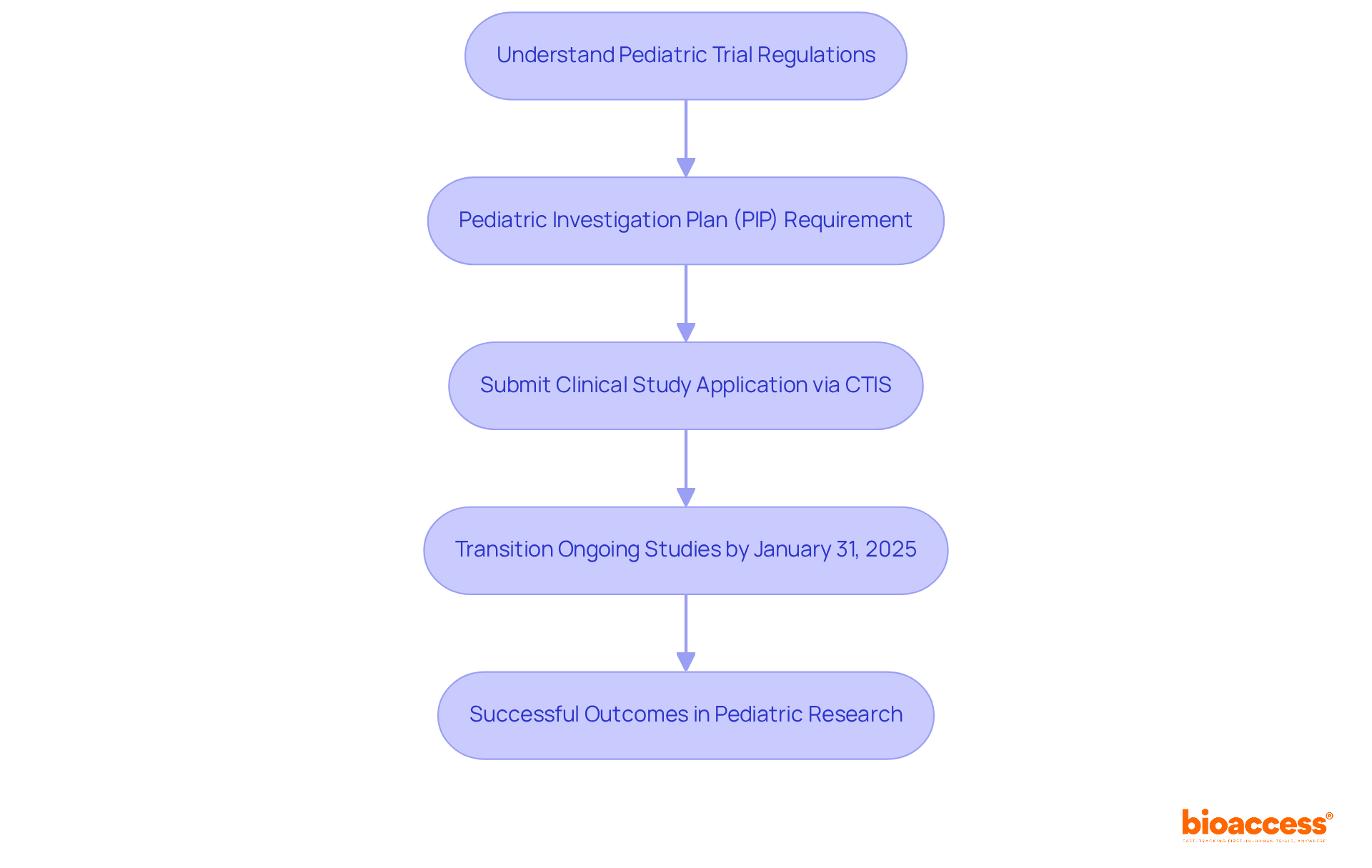
Navigating the pediatric trial authorization process in Bulgaria requires a solid understanding of the relevant regulations. The European Union's Clinical Studies Regulation (EU No 536/2014) governs clinical studies across member states, including Bulgaria. This regulation mandates a Pediatric Investigation Plan (PIP) for studies involving children, ensuring that essential data is collected to assess the safety and efficacy of treatments for pediatric populations. Notably, pediatric studies account for approximately 18.8% of all research in the surveyed EU-EECs, underscoring the importance of this area of investigation.
The Bulgarian Drug Agency plays a crucial role in overseeing the implementation of these regulations, ensuring compliance with both national and EU standards. Recent updates, such as the mandatory submission of clinical study applications through the Clinical Trials Information System (CTIS), which took effect on January 31, 2023, simplify the process significantly. All ongoing clinical studies must transition to the CTIS by January 31, 2025, highlighting the urgency of grasping these regulations. Being familiar with these updates will prepare you for the next steps in the pediatric trial authorization process in Bulgaria.
As noted by Cromos Pharma, 'Our team has met or reduced enrollment timelines in 95% of the conducted studies.' This statement reinforces the effectiveness of the regulatory environment in Bulgaria, showcasing the potential for successful outcomes in pediatric clinical research.
Preparing the necessary documentation is a critical step in the pediatric study authorization process. Key documents include:
By meticulously preparing these documents, you can facilitate a smoother approval process and demonstrate your commitment to ethical research practices.

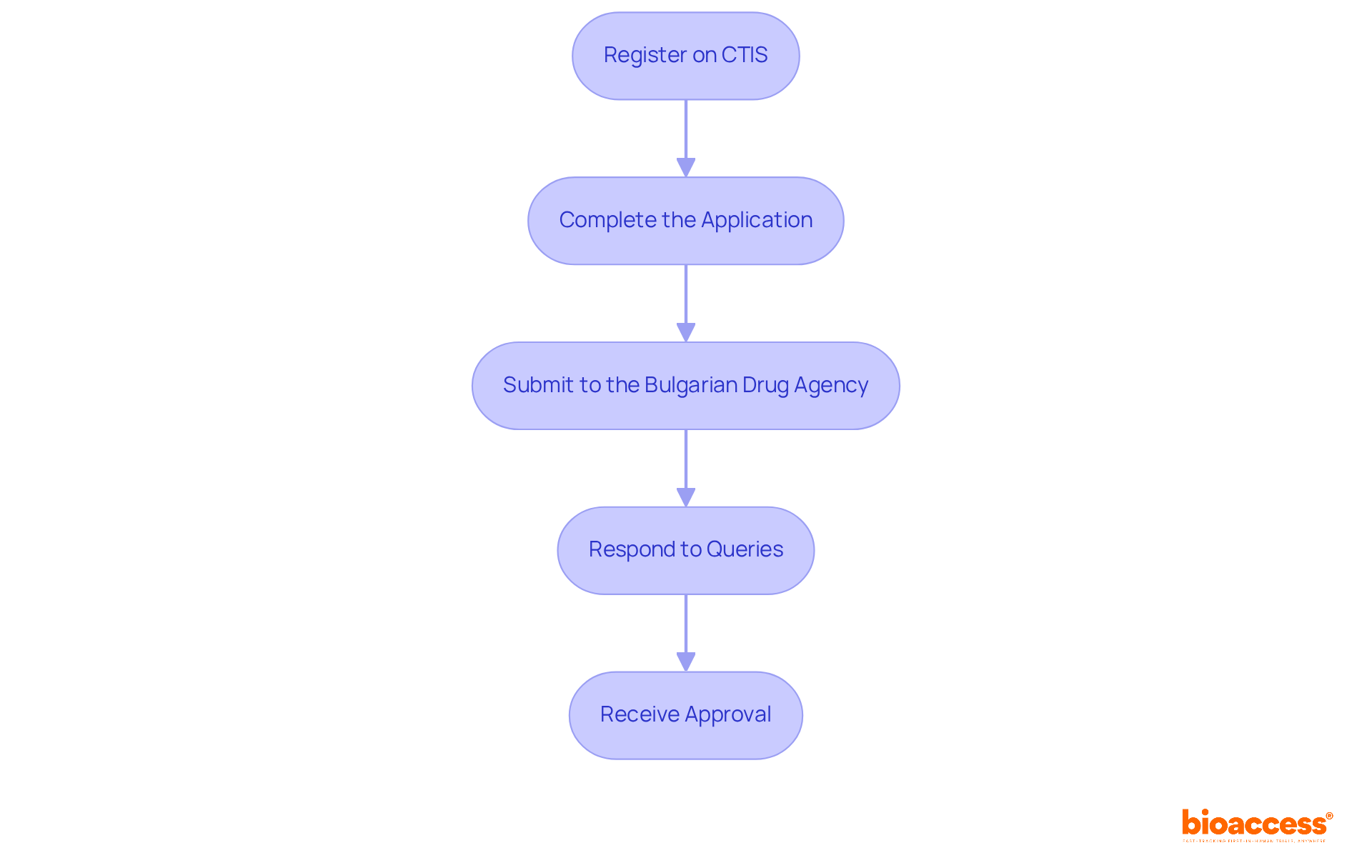
Once your documentation is prepared, the next step is to submit your applications. Follow these steps:
Effectively managing the pediatric trial authorization process in Bulgaria is essential for upholding timelines and ensuring adherence to legal expectations. This is particularly crucial given Bulgaria's increasing reputation as a desirable site for clinical studies, attributed to its high number of practicing doctors and unaddressed medical needs.
Ensuring adherence during the implementation of your pediatric study is crucial for protecting participant safety and upholding information integrity. Here are key compliance measures to follow:
Regular Monitoring: Establish a comprehensive monitoring plan that includes frequent site visits and audits to verify adherence to the trial protocol and regulatory standards. Regular monitoring is crucial, as it helps identify deviations early and ensures that corrective actions are taken promptly.
Information Management: Follow strict information management practices that align with privacy protection regulations, especially the General Data Protection Regulation (GDPR). This includes maintaining accurate, secure records of all trial-related data and implementing robust data cleaning processes to minimize errors and enhance data quality.
Adverse Event Reporting: Develop a clear protocol for reporting adverse events. All serious adverse events must be communicated to regulatory authorities and ethics committees within specified timelines to ensure participant safety and regulatory compliance.
Informed Consent Updates: If there are any changes to the study protocol or associated risks, promptly update the informed consent forms and re-consent participants as necessary. This practice is vital for maintaining transparency and trust with participants.
Final Reporting: Upon study completion, submit comprehensive results to the Bulgarian Drug Agency and the European Medicines Agency (EMA), including findings specific to the pediatric population. This final reporting is critical for regulatory review and future research considerations.
By implementing these compliance measures, you can effectively navigate the complexities of the pediatric trial authorization process in Bulgaria, while ensuring both the welfare of participants and the integrity of your research.

Understanding the pediatric trial authorization process in Bulgaria is crucial for researchers dedicated to enhancing the safety and efficacy of treatments for children. By mastering the complexities of the regulatory landscape, including the requirements established by the European Union and the Bulgarian Drug Agency, researchers can significantly improve their chances of achieving successful study outcomes.
Key components such as the Pediatric Investigation Plan (PIP), Clinical Trial Application (CTA), and securing ethics committee approval form the foundation for navigating this process. Moreover, the recent transition to the Clinical Trials Information System (CTIS) simplifies submissions, making it essential for researchers to remain informed and compliant with these evolving regulations. Upholding rigorous compliance during the trial execution phase not only ensures participant safety but also preserves the integrity of research data.
In summary, the pediatric trial authorization process in Bulgaria presents both challenges and opportunities. By meticulously preparing the necessary documentation and adhering to compliance measures, researchers can facilitate smoother approvals and make meaningful contributions to pediatric healthcare. The importance of this process transcends regulatory compliance; it represents a vital step toward advancing medical knowledge and enhancing treatment options for children. Engaging in this area of research is not merely a professional obligation; it is a commitment to the well-being of future generations.
What regulations govern pediatric trials in Bulgaria?
Pediatric trials in Bulgaria are governed by the European Union's Clinical Studies Regulation (EU No 536/2014), which mandates a Pediatric Investigation Plan (PIP) for studies involving children.
What is the purpose of the Pediatric Investigation Plan (PIP)?
The Pediatric Investigation Plan (PIP) ensures that essential data is collected to assess the safety and efficacy of treatments for pediatric populations.
How significant are pediatric studies in the overall research landscape in the EU?
Pediatric studies account for approximately 18.8% of all research in the surveyed EU-EECs, highlighting their importance.
What role does the Bulgarian Drug Agency play in pediatric trials?
The Bulgarian Drug Agency oversees the implementation of pediatric trial regulations, ensuring compliance with both national and EU standards.
What recent updates have been made to the clinical trial application process in Bulgaria?
A recent update requires the mandatory submission of clinical study applications through the Clinical Trials Information System (CTIS), which took effect on January 31, 2023.
What is the deadline for ongoing clinical studies to transition to the CTIS?
All ongoing clinical studies must transition to the CTIS by January 31, 2025.
How effective is the regulatory environment in Bulgaria for pediatric clinical research?
According to Cromos Pharma, their team has met or reduced enrollment timelines in 95% of the conducted studies, indicating the effectiveness of the regulatory environment in Bulgaria for successful outcomes in pediatric clinical research.