


Mastering the timeline expectations for Clinical Trial Applications (CTAs) is not just important; it’s a critical endeavor that can dictate the success of innovative medical products entering the market. The landscape is shaped by varying regulatory requirements across jurisdictions, making it essential for sponsors to understand these nuances for efficient approvals.
How can stakeholders effectively navigate these complexities? Ensuring submissions are timely and compliant with all necessary regulations is paramount.
To effectively master the timeline expectations for CTA review, understanding the regulatory requirements governing these proposals is crucial. Each jurisdiction has its own regulations, and familiarity with these can significantly influence the speed and success of your application. Key regulations include:
FDA Regulations: In the United States, the FDA enforces a 30-day review period for Investigational New Drug (IND) applications, mirroring the initial assessments for CTAs. This timeline is essential for organizing submissions efficiently, as the FDA may request further information during this period, potentially prolonging the evaluation.
European Union Regulations: The EU Clinical Trials Regulation (EU) 536/2014 mandates a maximum evaluation period of 60 days for CTAs. Understanding the timeline expectations for CTA review enables sponsors to anticipate possible delays and prepare accordingly, ensuring that all required documentation is in order to facilitate a smooth evaluation.
Local Regulations: Each region may impose unique requirements, such as additional documentation or specific ethical approvals. Engaging with local governing entities early in the process can provide valuable insights into these requirements, helping to streamline the filing procedure.
By thoroughly grasping these governance frameworks, sponsors can navigate the complexities of CTA filings more efficiently, ensuring compliance and reducing the likelihood of delays in the approval process. Successful case studies demonstrate that proactive engagement with governing bodies and meticulous preparation of documentation can lead to expedited approvals, ultimately accelerating the path to market for innovative medical products.
Effective planning of application strategies and documentation is crucial for a seamless Clinical Trial Application (CTA) process. Here are essential practices to consider:
By applying these strategies, sponsors can significantly enhance their application process, leading to faster approvals and more effective clinical trial initiation.
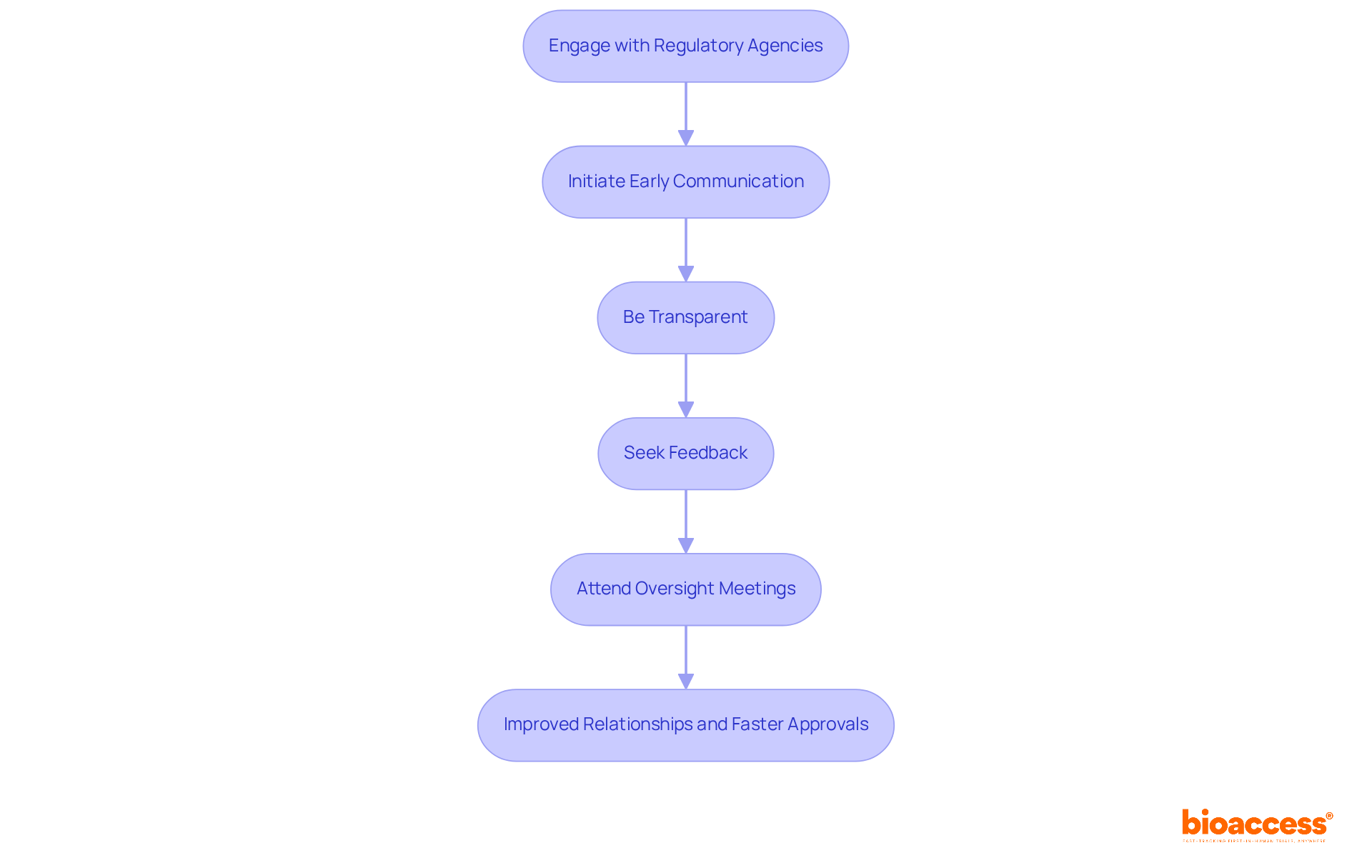
Effective engagement with oversight agencies is essential for expediting the Clinical Trial Application (CTA) review process. By implementing strategic practices, sponsors can foster positive relationships that lead to more efficient evaluations and faster approvals.
Initiate Early Communication: Start by reaching out to regulatory agencies early on to discuss your study design and submission strategy. This timely involvement clarifies expectations and identifies potential issues before submission, significantly impacting the timeline expectations for CTA review.
Be Transparent: Keep open lines of communication with regulators by providing regular updates on your study and any changes to your submission. Transparency builds trust and facilitates smoother interactions, which is crucial for compliance.
Seek Feedback: After submitting your CTA, proactively request input from governing bodies. This approach demonstrates a commitment to compliance and helps address any concerns they may have, ultimately enhancing the review process.
Attend Oversight Meetings: Engage in meetings or workshops organized by governing bodies. These events offer valuable insights into oversight expectations and allow for direct interaction with agency representatives, further strengthening relationships.
By applying these engagement strategies, sponsors can significantly improve their relationships with regulatory agencies, paving the way for more efficient evaluations and expedited approvals.
Monitoring and adjusting the timeline expectations for CTA review is crucial for keeping the Clinical Trial Application (CTA) on track. By implementing best practices, you can significantly enhance efficiency in this vital process:
By actively monitoring and adapting timelines, sponsors can enhance their ability to meet timeline expectations for CTA review and secure timely approvals.
Mastering the timeline expectations for Clinical Trial Applications (CTAs) is crucial for sponsors who want to navigate the complex regulatory landscape effectively. By grasping the specific requirements and timelines set by regulatory bodies like the FDA and the European Union, sponsors can streamline their submission processes and significantly enhance their chances of timely approvals.
Key strategies highlighted throughout this article include:
Developing a detailed submission plan, utilizing checklists, and fostering transparent communication with oversight bodies can greatly improve the likelihood of success. Moreover, monitoring progress and being ready to adapt timelines based on feedback helps minimize potential delays.
Ultimately, the path to a successful CTA review is paved with proactive planning, strategic engagement, and ongoing monitoring. By implementing these best practices, sponsors not only refine their submission processes but also contribute to the timely delivery of innovative medical solutions to the market. Embracing these principles leads to a more efficient and effective clinical trial landscape, benefiting both sponsors and patients alike.
What are the key regulatory requirements for Clinical Trial Applications (CTAs)?
Key regulatory requirements for CTAs include FDA regulations in the United States, which enforce a 30-day review period for Investigational New Drug (IND) applications, and the European Union's Clinical Trials Regulation (EU) 536/2014, which mandates a maximum evaluation period of 60 days for CTAs.
How does the FDA's review period for CTAs affect submissions?
The FDA enforces a 30-day review period for IND applications, which is crucial for organizing submissions efficiently. During this period, the FDA may request further information, which can prolong the evaluation process.
What is the evaluation period for CTAs in the European Union?
The EU Clinical Trials Regulation (EU) 536/2014 mandates a maximum evaluation period of 60 days for CTAs, allowing sponsors to anticipate possible delays and prepare necessary documentation accordingly.
What local regulations should sponsors be aware of when submitting CTAs?
Local regulations may impose unique requirements, such as additional documentation or specific ethical approvals. Engaging with local governing entities early in the process can provide valuable insights into these requirements, helping to streamline the filing procedure.
How can sponsors ensure a smoother CTA approval process?
Sponsors can ensure a smoother CTA approval process by thoroughly understanding regulatory frameworks, proactively engaging with governing bodies, and meticulously preparing documentation to facilitate compliance and reduce delays.
Real-World Evidence: Best Practices for Successful Regulatory Engagements - ACRP (https://acrpnet.org/2025/02/14/real-world-evidence-best-practices-for-successful-regulatory-engagements)
Best Practices
Case Studies (https://ors.od.nih.gov/OD/OQM/benchmarking/bestpractice/Pages/case_studies.aspx)
Strategies for Meaningful, Effective and Compliant Medical Communication in 2025 (https://lifesciences.enago.com/blogs/strategies-for-meaningful-effective-and-compliant-medical-communication-in-2025)
3 quotes about regulatory affairs that will make you smile | Regulatory Affairs Professionals Society (RAPS) (https://linkedin.com/posts/regulatory-affairs-professionals-society-raps-_3-quotes-about-regulatory-affairs-that-will-activity-7370109430329409536-T7xL)