This article outlines the essential steps for successful Master Tracker registration, underscoring the critical importance of understanding regulatory requirements and accurately completing the application process. It details the necessary documentation and highlights the role of INVIMA as the governing body in Colombia. Additionally, it provides common troubleshooting tips to address potential issues during registration. Thorough preparation is emphasized as a key factor in preventing delays and enhancing compliance, ensuring a smoother registration experience.
Navigating the complexities of tracker registration can be daunting, particularly given the constantly shifting regulatory landscape governing health products. Understanding the requirements and processes is crucial—not only does it ensure compliance, but it also paves the way for successful product approval and market entry. Many, however, encounter challenges that lead to delays and frustration. How can these hurdles be effectively overcome to streamline the registration journey?
This guide explores essential steps and strategies for mastering tracker registration, transforming compliance into a catalyst for innovation rather than a barrier. By delving into the intricacies of the Medtech landscape, we aim to provide insights that empower you to navigate these challenges with confidence.
Before you embark on the tracker registration procedure, it is crucial to grasp the specific requirements and prerequisites. Here’s how to navigate this process effectively:
Identify the Regulatory Body: Determine which regulatory organization governs the registration process in your field—be it Medtech, Biopharma, or Radiopharma. In Colombia, this is INVIMA (Instituto Nacional de Vigilancia de Medicamentos y Alimentos), the authority responsible for overseeing health products, including medical devices. Recognized as a Level 4 health authority by the Pan American Health Organization/World Health Organization, INVIMA upholds stringent standards for safety, efficacy, and quality.
Gather Necessary Documentation: Assemble all required documents, such as:
Review Guidelines: Familiarize yourself with the guidelines set forth by the governing authority. This includes understanding specific forms, associated fees, and submission timelines.
Check for Additional Requirements: Be mindful that some jurisdictions may impose extra prerequisites, like ethical approvals or submissions to an Institutional Review Board (IRB). Ensure these are secured before moving forward.
Consult with Experts: If any requirements seem unclear, seek advice from a clinical research management expert or legal advisor to clarify uncertainties. Katherine Ruiz, a specialist in compliance matters for medical devices and in vitro diagnostics in Colombia, can provide valuable insights into navigating these complexities.
Understanding these prerequisites is vital; non-compliance can lead to significant delays and potential fines. For example, the average time for 510(k) applications can stretch to five months due to requests for additional information, highlighting the need for thorough preparation. Moreover, around 67% of submissions face requests for more information, underscoring the commonality of this issue and the necessity for proactive measures. By establishing a robust tracker registration system for enrollment deadlines, clinical research directors can enhance adherence and streamline interactions with oversight bodies. Additionally, manufacturers must register their establishments with the FDA annually under 21 CFR 807 to maintain compliance, further emphasizing the importance of understanding the regulatory landscape. As Ana Criado aptly notes, "Viewing compliance as a strategic component can convert challenges into opportunities for innovation and growth.
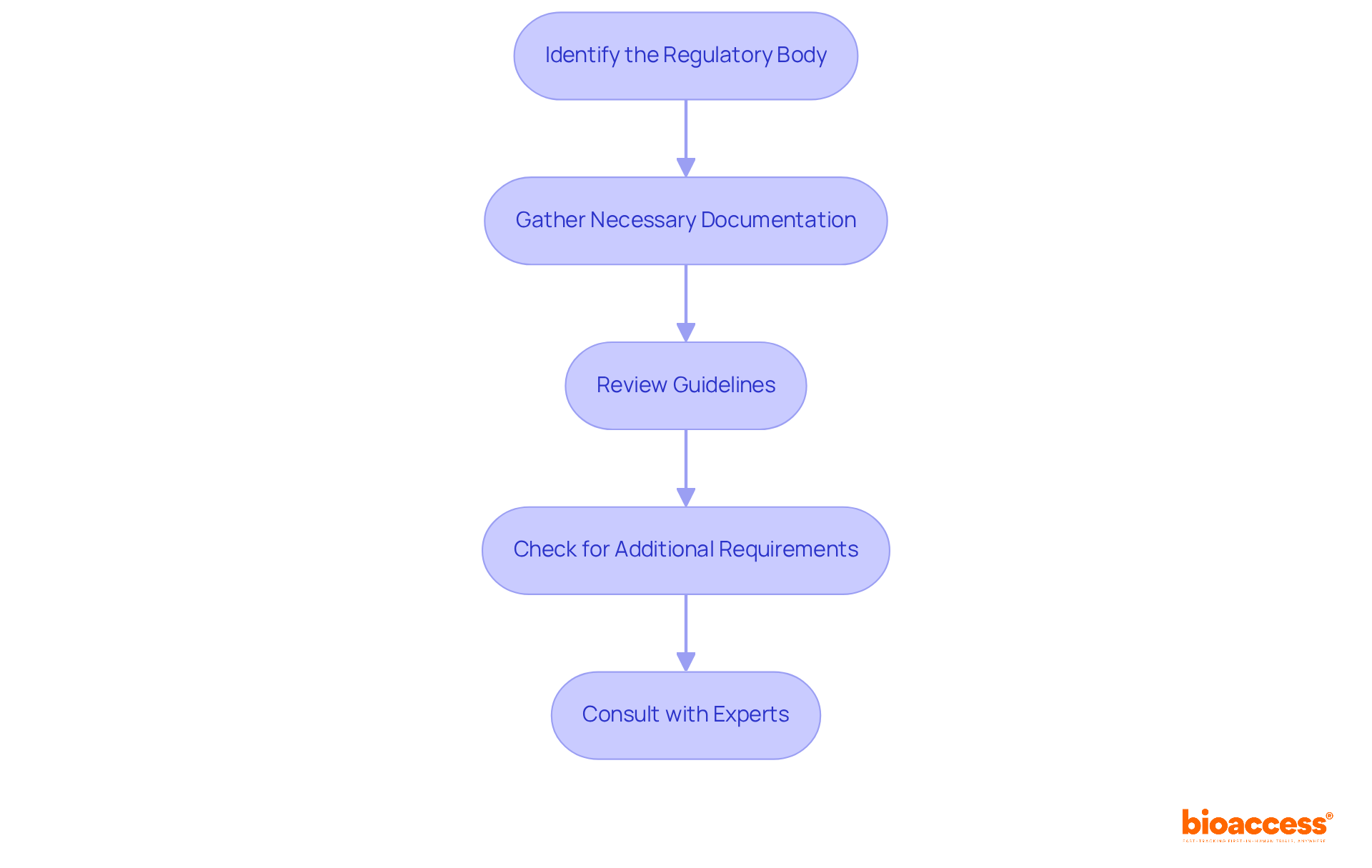
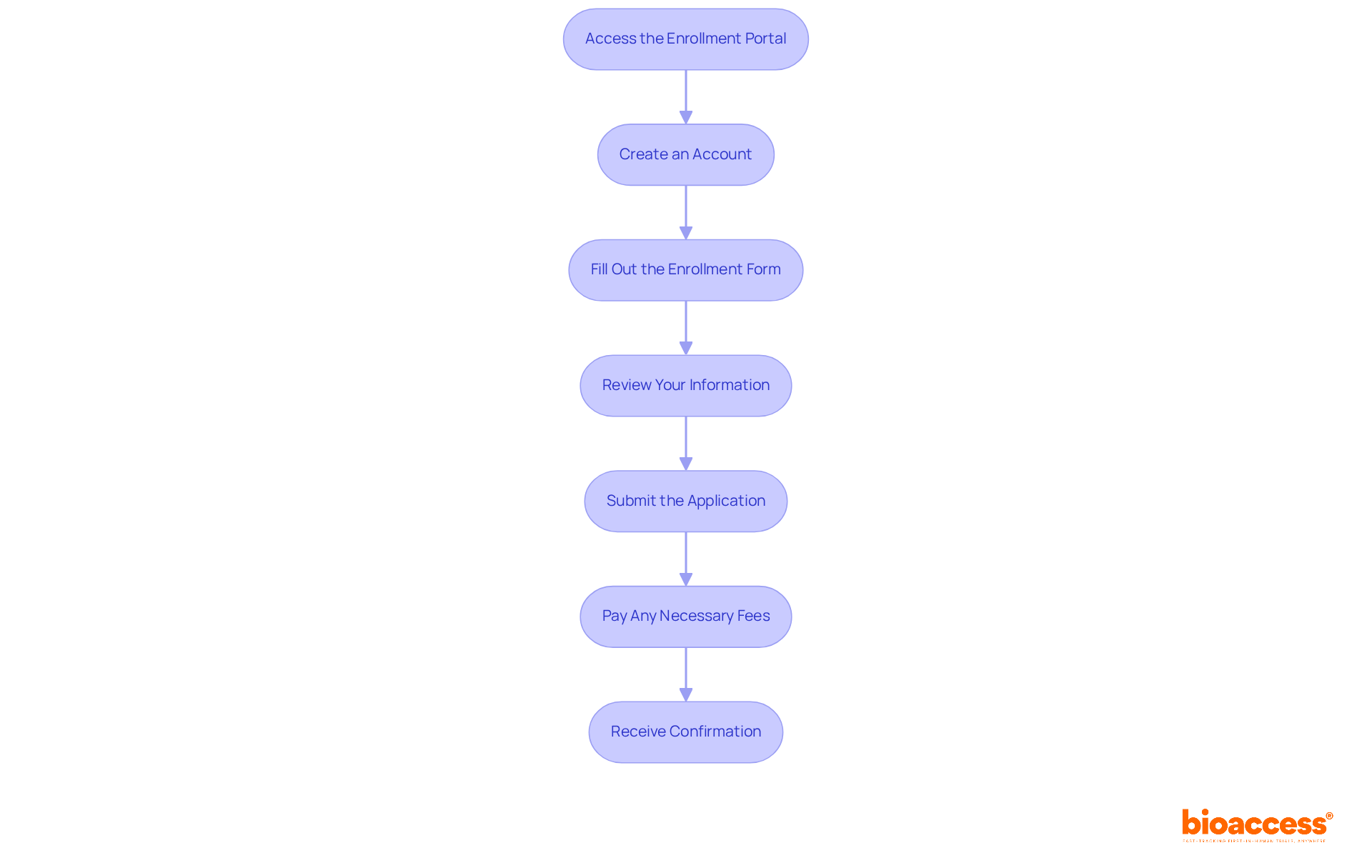
To successfully complete the tracker registration process, follow these essential steps:
Understanding INVIMA's role as a Level 4 health authority is crucial. This ensures that your tracker registration complies with the necessary standards for the safety and effectiveness of medical devices in Colombia.
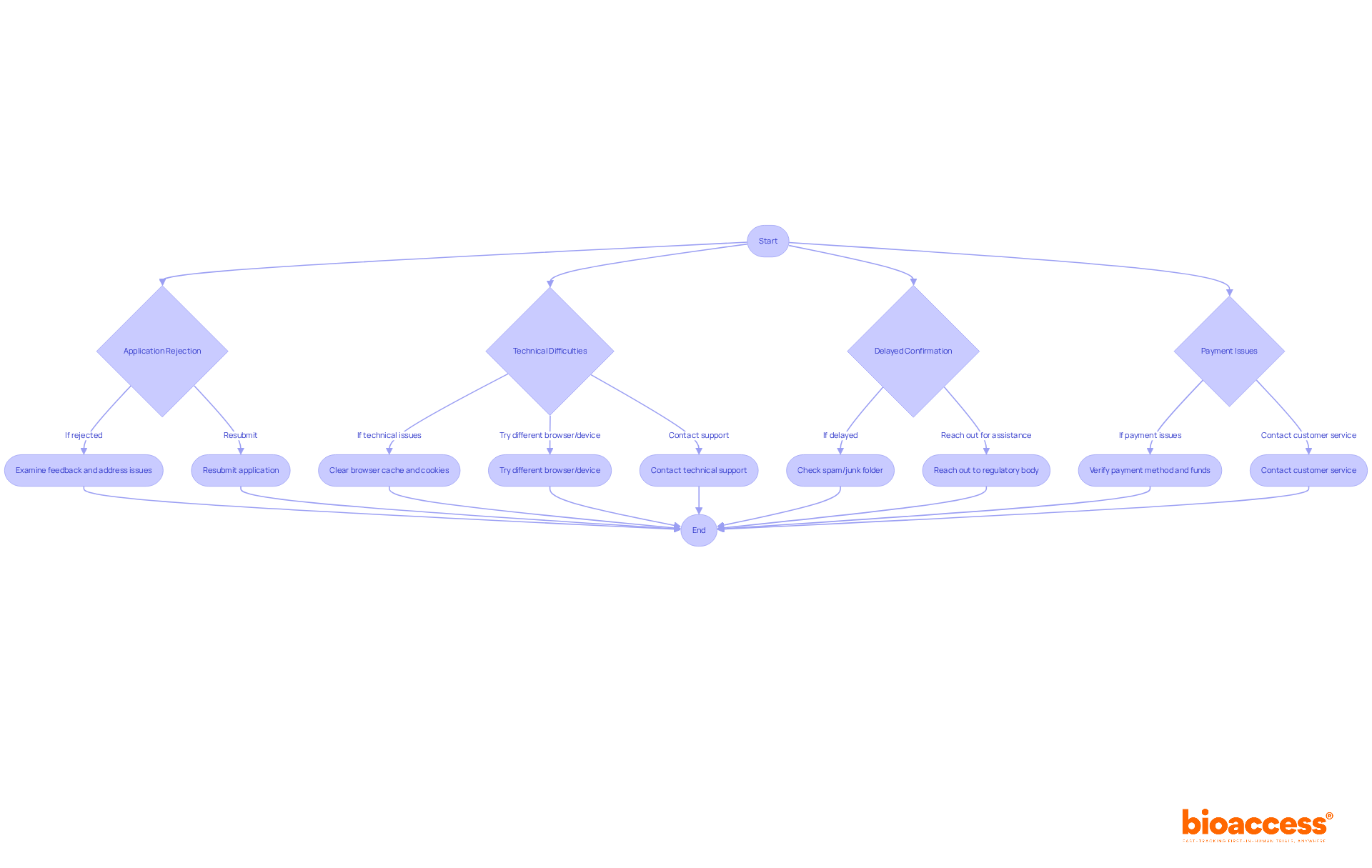
Even with careful preparation, issues may arise during the sign-up process. Here’s how to troubleshoot common problems:
Application Rejection: If your application is denied, examine the feedback provided by the governing agency. Common reasons include incomplete documentation, incorrect information, and missing signatures. Address these issues and resubmit promptly.
Technical Difficulties: If you encounter technical issues with the tracker registration portal, clear your browser cache and cookies. Try using a different browser or device, and contact technical support if problems persist.
Delayed Confirmation: If you do not receive a confirmation email within the expected timeframe, check your spam or junk folder. Ensure that your email address was entered correctly during registration, and reach out to the regulatory body for assistance.
Payment Issues: If you experience problems with payment, verify that your payment method is valid and ensure that you have sufficient funds. Contact customer service for the registration portal if issues continue.
Navigating the complexities of Master Tracker registration is crucial for ensuring compliance and achieving success in the health product sector. Understanding the registration requirements and effectively managing the process allows stakeholders to position themselves advantageously while adhering to the stringent standards set by regulatory bodies like INVIMA.
Key steps include:
Addressing potential challenges—such as application rejections or technical issues—further underscores the importance of thorough preparation. Engaging with experts and utilizing available resources can significantly streamline the process and help mitigate common pitfalls.
Ultimately, the significance of Master Tracker registration cannot be overstated. It serves not only as a compliance measure but also as a strategic opportunity for innovation and growth within the industry. By taking proactive steps and viewing compliance as an integral part of the operational framework, organizations can transform challenges into avenues for advancement. Embracing these principles ensures that the registration process is not merely a requirement, but a pathway to success.
What are the first steps to take before starting the tracker registration process?
Before starting the tracker registration process, it is important to understand the specific requirements and prerequisites, identify the regulatory body governing the registration in your field, and gather all necessary documentation.
Which regulatory body oversees the registration process in Colombia?
In Colombia, the regulatory body responsible for overseeing the registration process is INVIMA (Instituto Nacional de Vigilancia de Medicamentos y Alimentos), which governs health products, including medical devices.
What types of documentation are required for tracker registration?
Required documentation includes proof of identity and qualifications, evidence of prior research experience or credentials, and relevant certifications or licenses.
Why is it important to review the guidelines set forth by the governing authority?
Reviewing the guidelines is crucial to understand specific forms, associated fees, and submission timelines, which helps ensure compliance and avoid delays in the registration process.
Are there any additional requirements that may need to be fulfilled?
Yes, some jurisdictions may impose extra prerequisites, such as ethical approvals or submissions to an Institutional Review Board (IRB), which should be secured before proceeding with the registration.
Who can I consult for clarification on registration requirements?
It is advisable to consult with a clinical research management expert or legal advisor. For instance, Katherine Ruiz specializes in compliance matters for medical devices and in vitro diagnostics in Colombia and can provide valuable insights.
What are the consequences of non-compliance during the registration process?
Non-compliance can lead to significant delays and potential fines. For example, the average time for 510(k) applications can extend to five months due to requests for additional information.
How common are requests for additional information during submissions?
Approximately 67% of submissions face requests for more information, highlighting the importance of thorough preparation and proactive measures to avoid delays.
What is the significance of establishing a robust tracker registration system?
Establishing a robust tracker registration system for enrollment deadlines can enhance adherence to regulatory requirements and streamline interactions with oversight bodies.
What annual requirement must manufacturers fulfill to maintain compliance with the FDA?
Manufacturers must register their establishments with the FDA annually under 21 CFR 807 to maintain compliance.