


Navigating the regulatory landscape for drug trials in Montenegro presents a significant challenge, characterized by complex requirements and essential documentation that must be meticulously prepared. This article serves as a comprehensive checklist, guiding researchers through the critical steps necessary for successful regulatory submissions. With the complexities involved, one must consider: how can all submissions not only meet local requirements but also uphold ethical standards? Understanding these nuances is vital for ensuring compliance and fostering trust in clinical research.
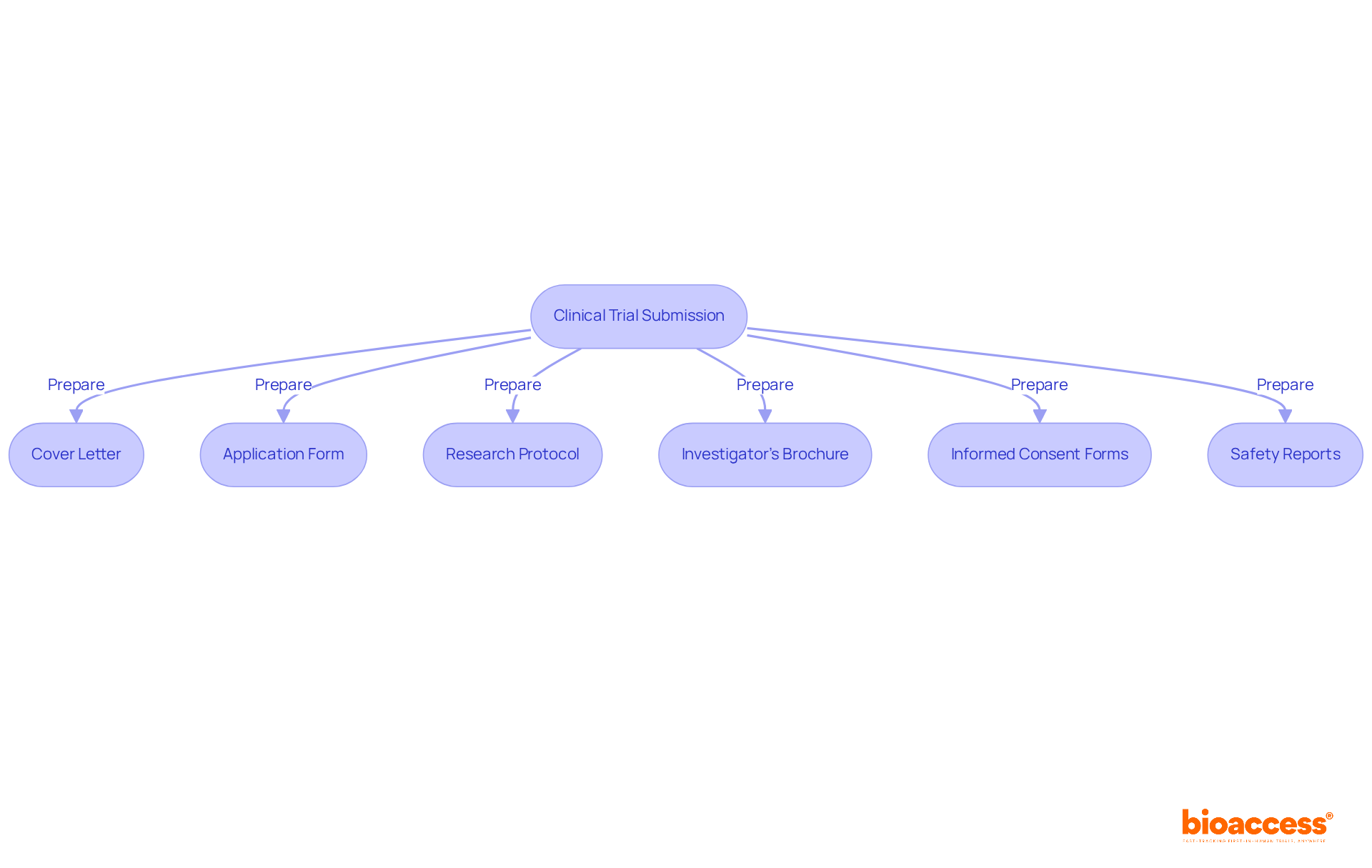
To ensure a successful submission for clinical trial approval in Montenegro, compiling the following documentation is essential:
At bioaccess, we specialize in comprehensive research study management services. Our offerings include feasibility assessments, site selection, compliance reviews, study setup, import permits, project management, and reporting. With our expertise, we ensure that all necessary documentation meets the regulatory submission checklist for Montenegro drug trials, facilitating a smooth approval process.
In the ever-evolving Medtech landscape, navigating the complexities of clinical research can be challenging. That's where bioaccess steps in, addressing key challenges with confidence and authority. Collaboration is vital, and we are here to support you every step of the way. Let's work together to achieve your clinical research goals.
Clinical Study Application (CTA): This application is essential for initiating clinical studies, ensuring that all necessary information is submitted to regulatory authorities for thorough evaluation.
Marketing Authorization Application (MAA): A pivotal phase in the drug development process, the MAA is required to obtain approval for selling the drug after successful testing. This submission must convincingly demonstrate the drug's safety and efficacy, backed by comprehensive research data.
Modifications: Any changes to the original protocol or research design must be submitted as modifications to keep the regulatory body informed and compliant with evolving parameters.
Annual Safety Reports: These reports are mandatory for updating the regulatory body on the safety profile of the investigational product, ensuring continuous monitoring of any adverse effects.
Final Study Reports: Upon completion of the study, final reports must be submitted, detailing outcomes and findings, which are crucial for evaluating the study's success and guiding future research directions.
In Colombia, the process also involves obtaining an import permit from the Ministry of Industry and Commerce (MinCIT) to ship investigational devices. This highlights the regulatory hurdles that medical device startups may encounter. Bioaccess® offers specialized services to tackle these challenges, ensuring expedited research studies for Medtech, Biopharma, and Radiopharma startups.

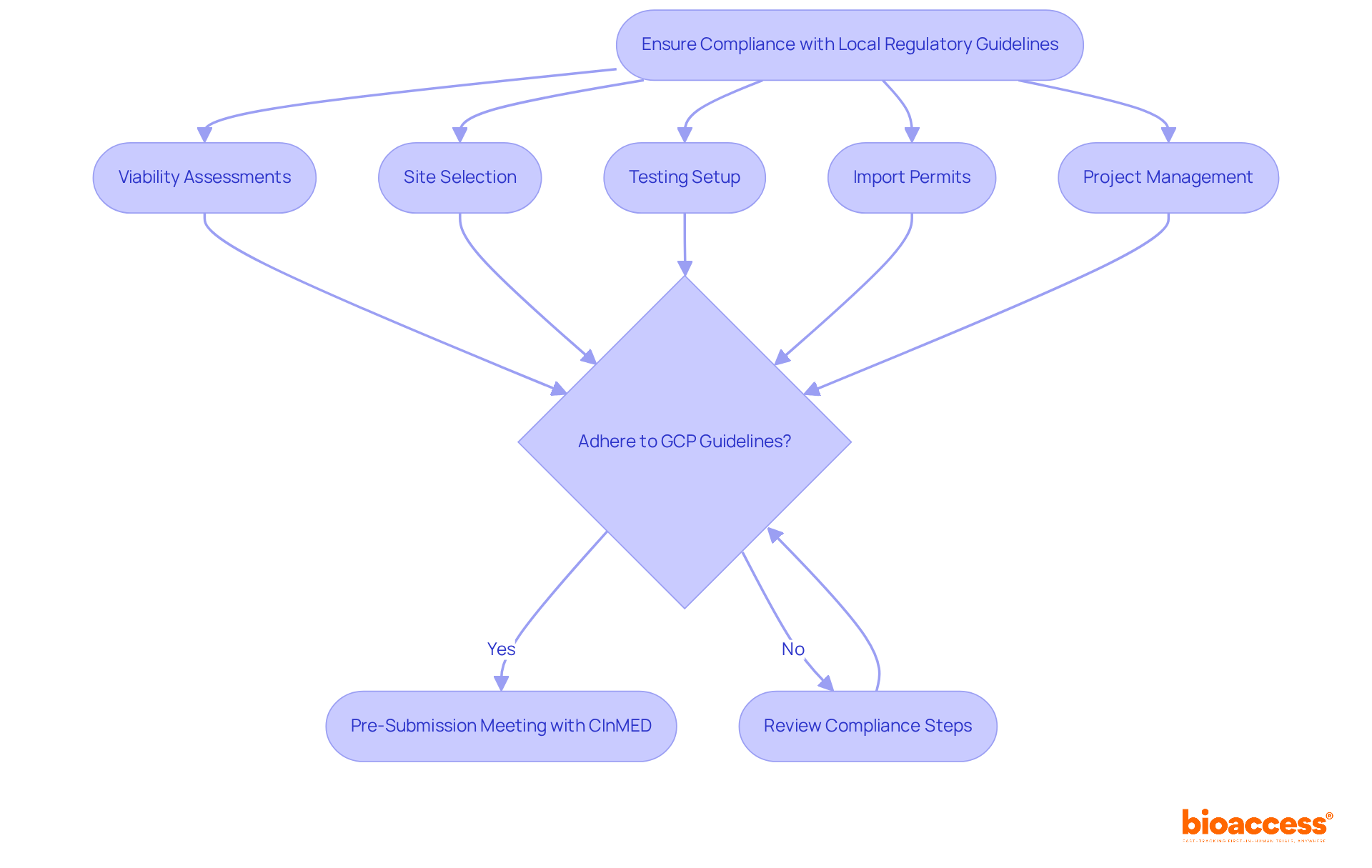
To ensure compliance with local regulatory guidelines, it’s essential to thoroughly examine the regulatory submission checklist for Montenegro drug trials, along with the Law on Medicines and related regulations. This understanding is crucial for navigating the legal landscape that governs research studies. Bioaccess stands ready to assist with comprehensive services, including:
Our goal is to ensure that all documentation aligns with the regulatory submission checklist for Montenegro drug trials, as set by the Agency for Medicines and Medical Devices (CInMED), which oversees the regulatory framework for research in the country.
Moreover, we guarantee that the experimental protocol adheres to Good Clinical Practice (GCP) guidelines, which are vital for maintaining the integrity and ethical standards of research studies. Why is GCP adherence so important? Research indicates that compliance rates for studies in Montenegro are on the rise, with a significant percentage meeting these global standards. Additionally, bioaccess ensures that all researchers and study sites are registered and comply with local regulations, confirming they possess the necessary qualifications and permissions to conduct clinical research.
If needed, we also facilitate a pre-submission meeting with CInMED to clarify any uncertainties about the regulatory submission checklist for Montenegro drug trials. This proactive approach helps streamline the approval process and addresses potential issues before formal submission. By collaborating with bioaccess, you can navigate the complexities of clinical research with confidence, ensuring that your studies not only comply with local regulations but also uphold the highest ethical standards.
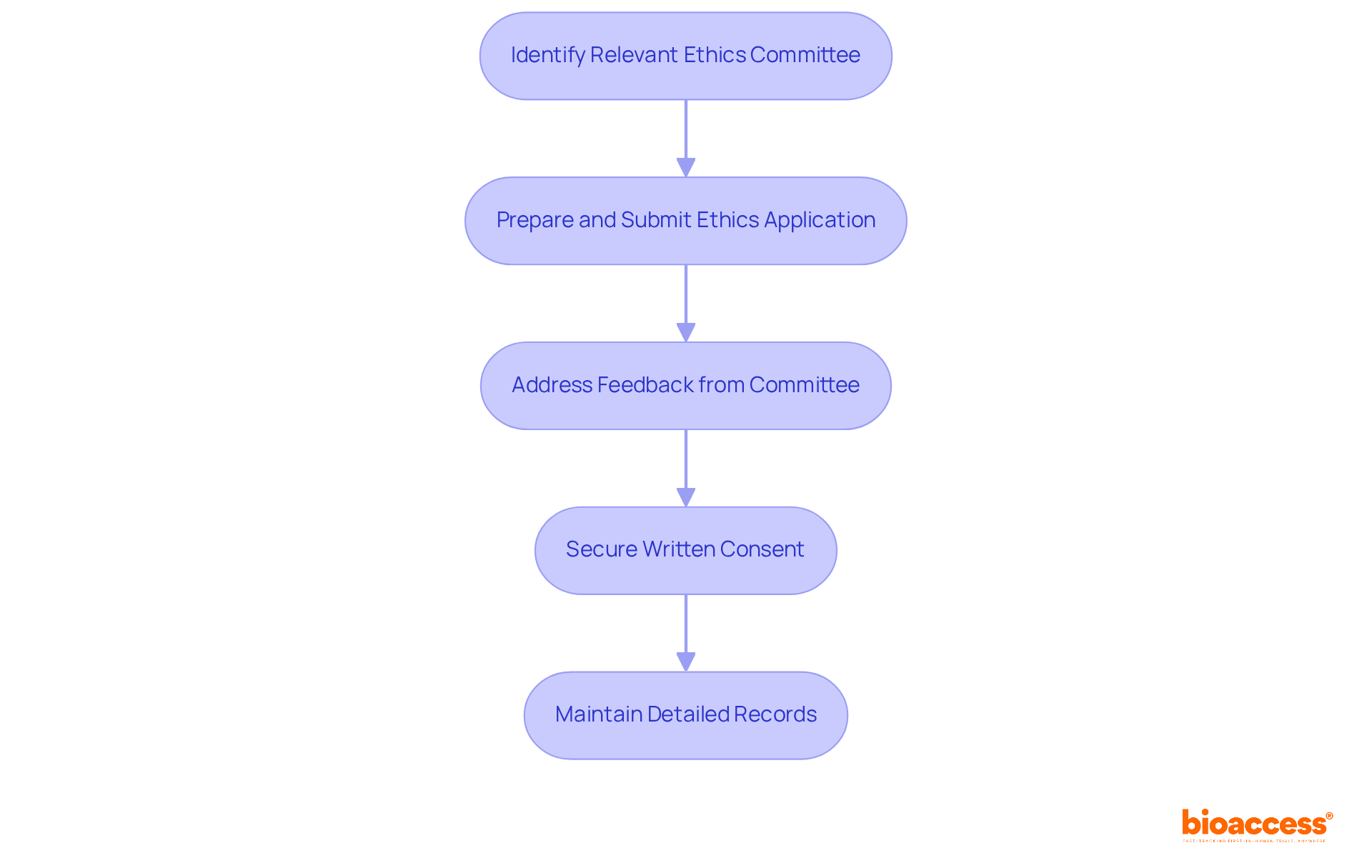
Identifying the relevant ethics committee for your trial is crucial, as it must align with the specific requirements of your research type. This step is not just a formality; it sets the foundation for your study's integrity and compliance with ethical standards. Once you've pinpointed the appropriate committee, prepare and submit your ethics application. This application should include essential documents such as the study protocol, informed consent forms, and any supplementary materials required by the committee.
Promptly addressing any feedback or requests for additional information from the ethics committee is vital. Delays in the authorization process can hinder your research timeline, so swift communication is key. Before commencing your study, ensure you secure written consent from the ethics committee. This is not merely a procedural step; it is a legal requirement outlined in the regulatory submission checklist for Montenegro drug trials involving human subjects.
Maintaining detailed records of all communications and consents is essential for future reference. These documents may be necessary for the regulatory submission checklist for Montenegro drug trials and audits, highlighting the importance of meticulous documentation.
In Montenegro, the average duration required to complete the regulatory submission checklist for Montenegro drug trials typically spans from 4 to 8 weeks. This timeframe can vary based on the complexity of the research and the responsiveness of the ethics committee. Generally, the application requirements include a detailed study protocol, informed consent documentation, and evidence of compliance with ethical standards. Successful ethics committee applications often highlight the significance of comprehensive preparation and transparent communication, which can greatly enhance the chances of acceptance.
Understanding the specific requirements and maintaining open lines of communication with the ethics committee are crucial for a smooth approval process. By prioritizing these elements, researchers can navigate the ethical landscape effectively, ensuring their studies are both compliant and ethically sound.
Navigating the regulatory landscape for drug trials in Montenegro demands a meticulous approach to ensure compliance and successful submission. Compiling essential documentation, understanding the types of regulatory submissions, and obtaining ethical approvals are critical steps that cannot be overlooked. Each phase of this process is vital in facilitating a smooth approval journey, ultimately contributing to the integrity and success of clinical research.
A comprehensive regulatory submission checklist is indispensable, encompassing vital documents such as the Clinical Study Application (CTA), Marketing Authorization Application (MAA), and annual safety reports. Adhering to local guidelines and securing the endorsement of ethics committees is paramount, highlighting the need for clear communication and thorough preparation throughout the submission process.
In summary, the regulatory submission checklist for Montenegro drug trials serves as a roadmap for researchers navigating the complexities of clinical trials. By prioritizing compliance and ethical standards, stakeholders can significantly enhance their chances of successful submissions and contribute to the advancement of medical research in Montenegro. Engaging with expert services like bioaccess can provide invaluable support, ensuring that every aspect of the submission process is addressed with precision and expertise.
What documentation is essential for clinical trial submission in Montenegro?
Essential documentation includes a cover letter, the finished application form for research approval, a detailed research protocol, the Investigator's Brochure (IB), informed consent forms, safety reports, and any relevant prior research data.
What should be included in the research protocol?
The research protocol must outline the experiment's objectives, methodology, and statistical analysis plan.
What is the purpose of the Investigator's Brochure (IB)?
The Investigator's Brochure provides detailed information about the investigational product being studied.
Why are informed consent forms necessary?
Informed consent forms are necessary to ensure ethical compliance by obtaining participants' agreement to participate in the study.
In what languages must the documents be prepared?
All documents must be prepared in either Montenegrin or English to comply with local regulations.
What services does bioaccess offer for clinical research study management?
Bioaccess offers services including feasibility assessments, site selection, compliance reviews, study setup, import permits, project management, and reporting.
How does bioaccess support the submission process for drug trials in Montenegro?
Bioaccess ensures that all necessary documentation meets the regulatory submission checklist, facilitating a smooth approval process for drug trials.