


The article emphasizes the critical importance of the Fragment Crystallizable Region (Fc region) in immunology, particularly regarding its pivotal role in antibody functionality and therapeutic applications. It asserts that the Fc region is indispensable for immune processes, including:
Furthermore, it highlights that modifications to this region can significantly enhance the pharmacokinetic properties of therapeutic proteins, thereby improving their efficacy in clinical settings. This underscores the need for ongoing research and collaboration to optimize therapeutic strategies in immunology.
The fragment crystallizable region (Fc region) of antibodies stands as a cornerstone of immunology, playing a vital role in the body’s immune response. This segment not only facilitates crucial interactions with immune cells but also significantly influences the efficacy of therapeutic proteins.
As researchers delve deeper into the complexities of the Fc region, critical questions emerge regarding how modifications can enhance treatment outcomes and improve drug delivery systems.
What implications do these advancements hold for the future of immunotherapy and patient care? The exploration of these questions is essential for advancing clinical research and improving patient outcomes.
The fragment crystallizable area, also known as the Fc segment, is a crucial element of an antibody molecule, located at its tail end. This segment comprises two identical heavy chains and differs from the Fab (Fragment Antigen Binding) segment, which contains a fragment crystallizable part responsible for binding to antigens. The Fc area, which includes the fragment crystallizable region, plays an essential role in the body's defense mechanisms, facilitating vital processes such as:
For instance, therapeutic antibodies like trastuzumab leverage the fragment crystallizable portion to effectively interact with immune defense cells, enhancing their ability to target and eliminate cancer cells. Recent research indicates that modifications to the Fc section can significantly improve the pharmacokinetic properties of proteins, particularly enhancing the fragment crystallizable region, which leads to improved treatment efficacy. Understanding the composition and function of the fragment crystallizable portion is fundamental for developing effective therapeutic proteins, as it directly influences their interactions with immune cells and overall clinical effectiveness.
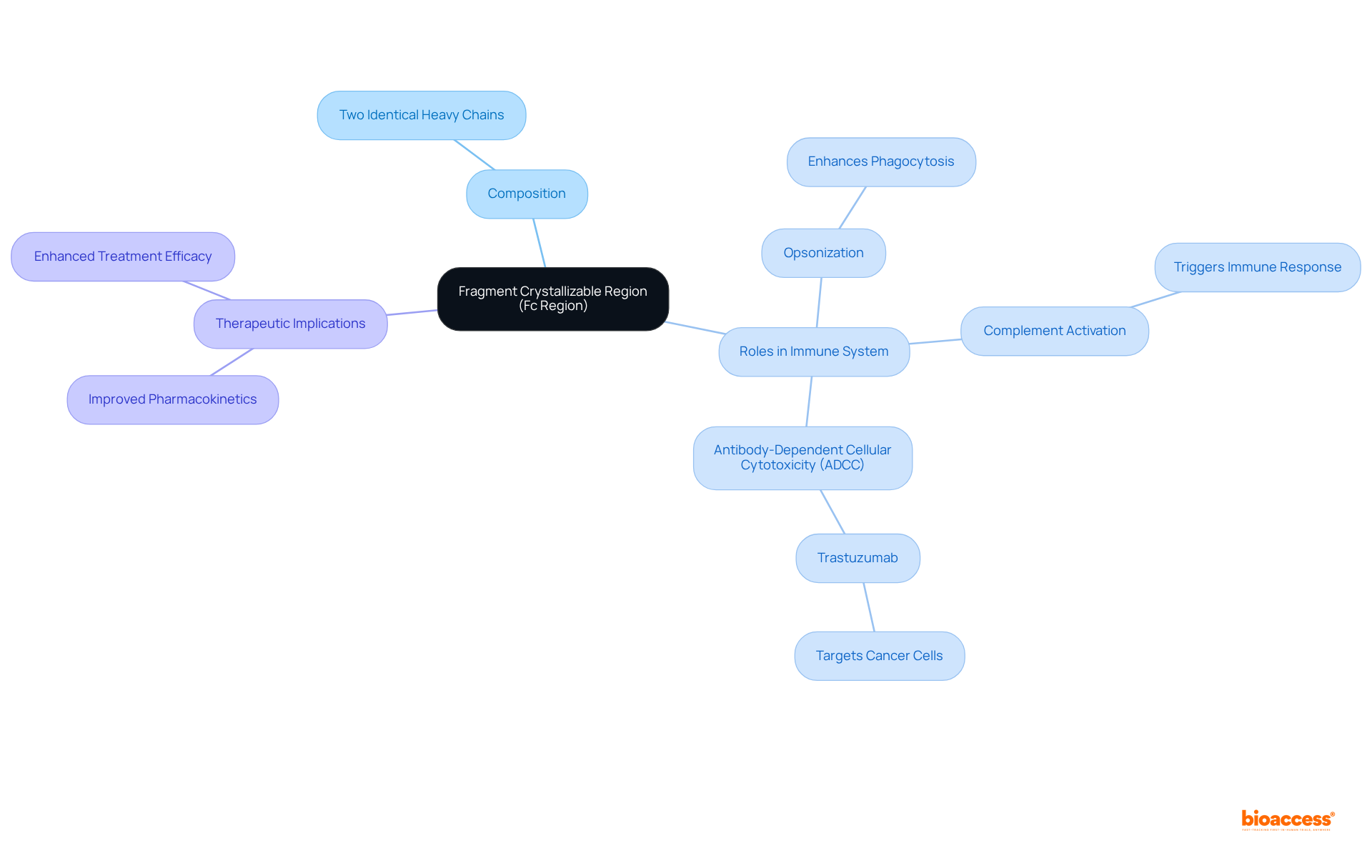
The Fc area is pivotal in the defense system, primarily interacting with Immunoglobulin G (IgG) and serum albumin. IgG antibodies exhibit a strong affinity for the Fc area, facilitating their effective attachment to Fc receptors found on defense cells, such as macrophages and natural killer (NK) cells. This interaction is essential for initiating immune responses, including phagocytosis and antibody-dependent cellular cytotoxicity (ADCC).
Recent research underscores that modifications to the Fc area can enhance these interactions, leading to improved treatment outcomes. For example, long-acting biologics utilize these Fc interactions to extend their half-lives in circulation, with IgG subclasses 1, 2, and 4 demonstrating half-lives exceeding three weeks.
Furthermore, the binding of the Fc region to serum albumin not only stabilizes medical proteins but also significantly reduces their clearance rates, making it a critical factor in the design of effective biologics. The neonatal Fc receptor (FcRn) is also instrumental in recycling IgG and safeguarding it from degradation, which is vital for maintaining IgG homeostasis.
Understanding these dynamics is crucial for refining therapeutic molecule design and enhancing their effectiveness in clinical applications.
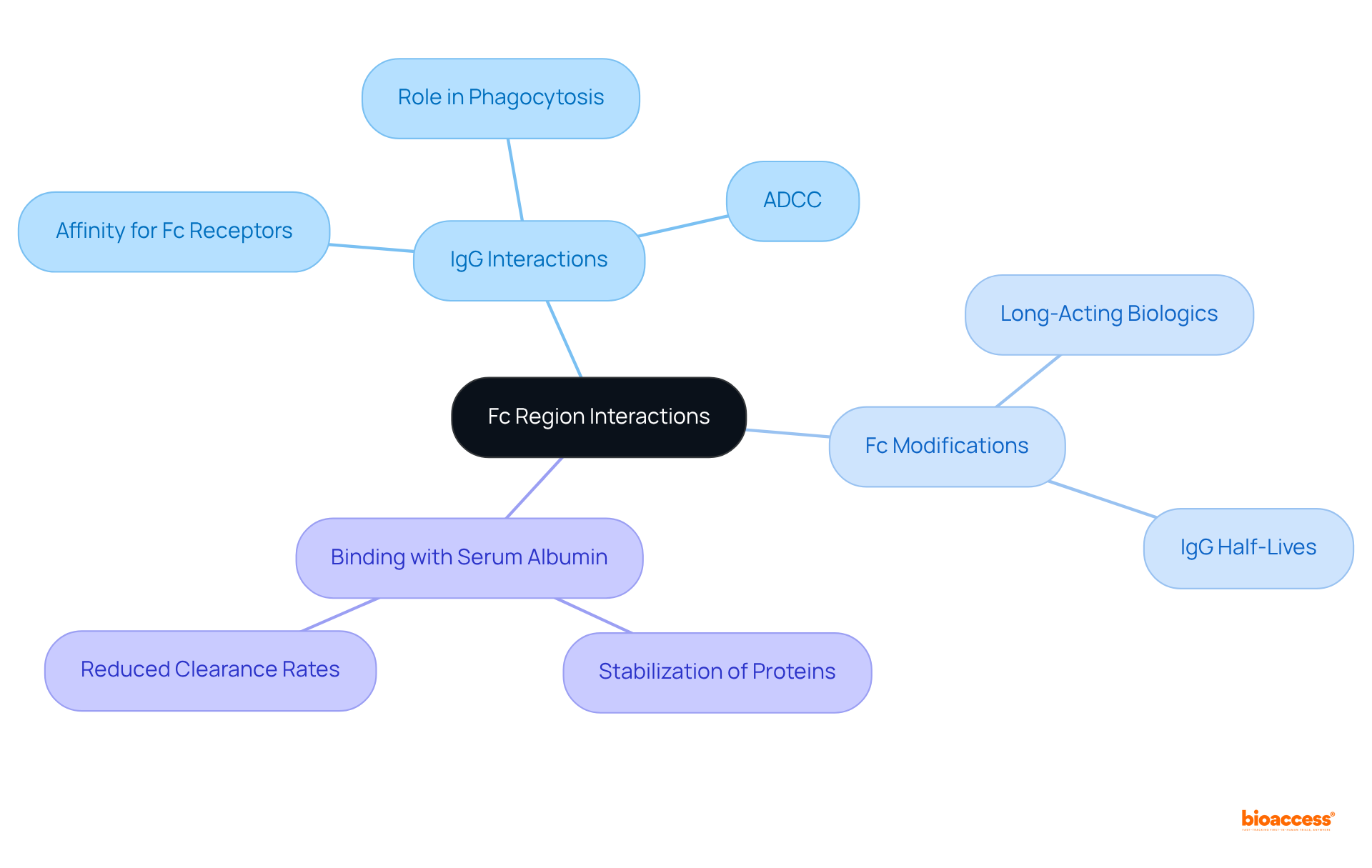
The neonatal Fc receptor (FcRn) serves a pivotal role in the recycling and transcytosis of immunoglobulins, particularly IgG. By attaching to the Fc portion of IgG in acidic environments, such as those found in endosomes, FcRn facilitates the reutilization of immune proteins back to the cell surface instead of their degradation. This recycling mechanism substantially prolongs the half-life of IgG in circulation. Furthermore, FcRn is integral to transcytosis, which involves the transport of immunoglobulins across epithelial barriers, including the intestinal epithelium and the blood-brain barrier. This capability is especially crucial for therapeutic antibodies that must access specific tissues or compartments within the body.
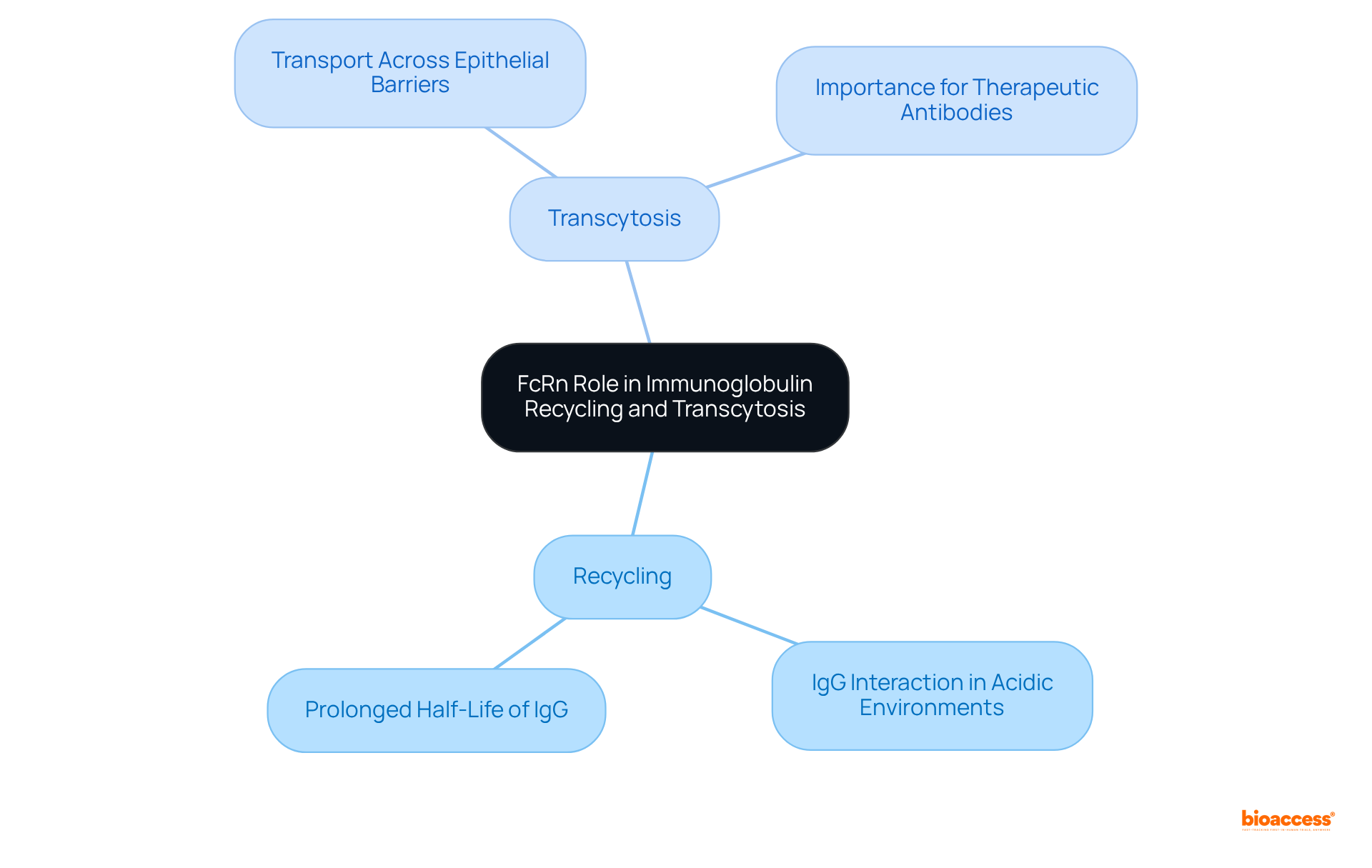
The Fc area serves multiple functions depending on the organ and the body's context. In the liver, for instance, the Fc section plays a pivotal role in the elimination of complexes and pathogens through its interactions with hepatic Fc receptors. In the lungs, the Fc portion facilitates the transfer of immunoglobulins across the airway epithelium, thereby providing localized immune defense. Moreover, in the placenta, the Fc segment is crucial for transferring maternal antibodies to the fetus, which is vital for ensuring neonatal immunity. Understanding these organ-specific functions is essential for developing targeted treatments that leverage the capabilities of the Fc area.
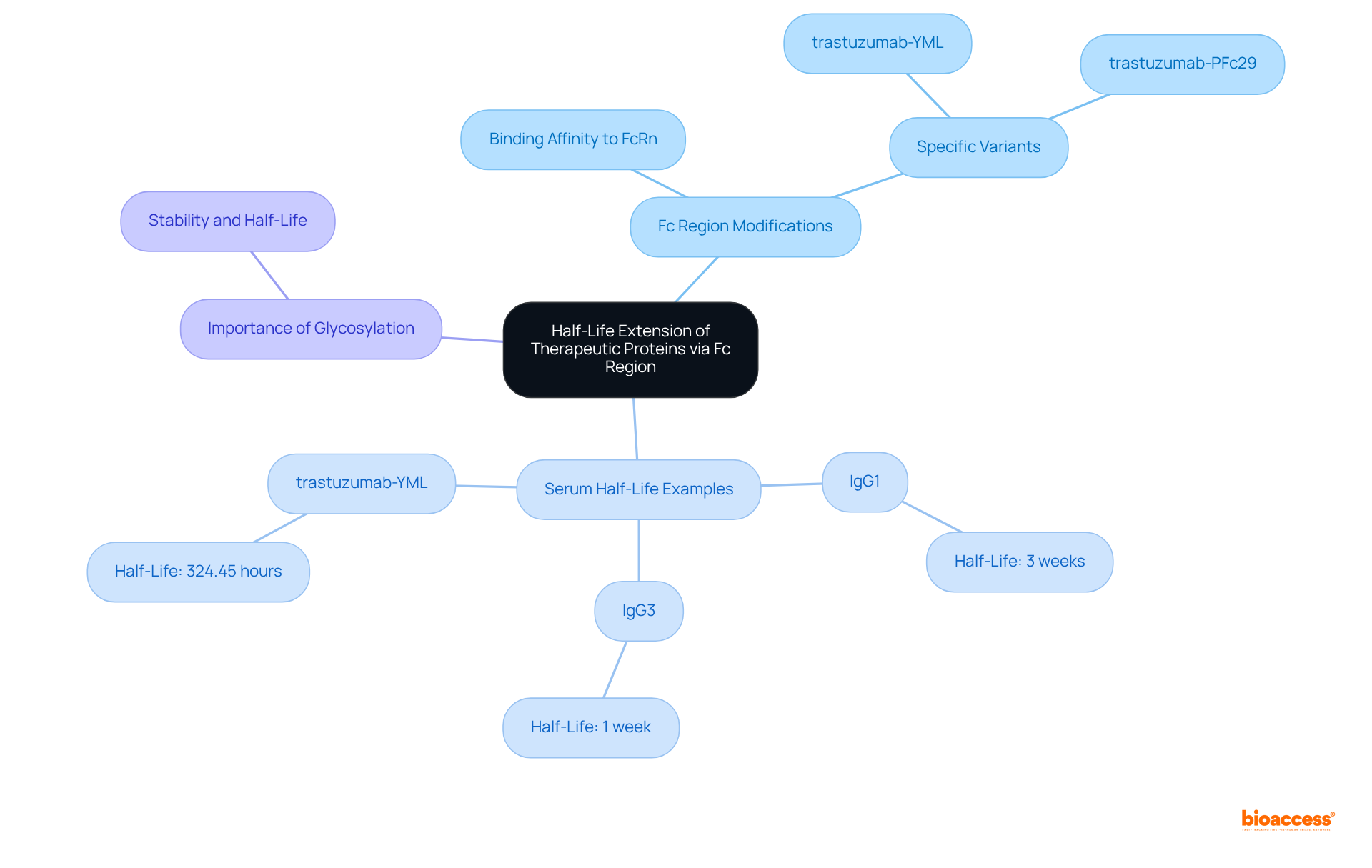
The half-life of treatment proteins is paramount for their efficacy and dosing regimens. Notably, alterations to the fragment crystallizable portion can significantly extend this half-life, thereby enhancing therapeutic outcomes. For instance, modifying the Fc portion to improve its binding affinity to the neonatal Fc receptor (FcRn) facilitates better recycling and reduces clearance rates. Recent studies reveal that variants such as trastuzumab-YML exhibit an impressive serum half-life of approximately 324.45 hours, underscoring the potential of Fc engineering to enhance drug availability.
Moreover, the fragment crystallizable region's glycosylation patterns are critical for protein stability and half-life; for example, the serum half-life of IgG3 is one week, compared to three weeks for IgG1. These strategies are particularly vital in the development of fragment crystallizable monoclonal antibodies and fusion proteins, where prolonged circulation time not only boosts treatment efficacy but also minimizes the frequency of administration, ultimately benefiting patient adherence and treatment effectiveness.
Ongoing research continues to investigate the effects of Fc region modifications, demonstrating that engineered variants can lead to substantial improvements in pharmacokinetics and therapeutic outcomes, establishing their significance in the dynamic landscape of antibody-based therapies.
The fragment crystallizable region (Fc region) stands as a cornerstone of antibodies, playing a pivotal role in the immune response and therapeutic applications. Its structure and function are indispensable for enhancing interactions with immune cells, facilitating critical processes such as opsonization, complement activation, and antibody-dependent cellular cytotoxicity. A comprehensive understanding of the Fc region is essential for the development of effective therapeutic proteins that can significantly improve clinical outcomes.
Key insights from this exploration reveal the Fc region's interactions with Immunoglobulin G (IgG) and serum albumin, which are vital for prolonging the half-life of therapeutic proteins. The neonatal Fc receptor (FcRn) further enhances this process by contributing to the recycling and transcytosis of IgG, ensuring that these antibodies retain their efficacy within the body. Moreover, the diverse roles of the Fc region across various organs underscore its importance in targeted therapies and the design of biologics.
The implications of these findings extend well beyond basic immunology, offering invaluable insights for the future of therapeutic development. As research continues to unveil the intricacies of the Fc region, it becomes imperative to consider its modifications and interactions in the design of next-generation therapies. By harnessing the unique properties of the Fc region, advancements in drug delivery systems and improved patient outcomes can be realized, solidifying its status as a cornerstone of modern immunotherapy.
What is the fragment crystallizable region (Fc region) in antibodies?
The fragment crystallizable region, or Fc region, is a crucial part of an antibody molecule located at its tail end. It consists of two identical heavy chains and is distinct from the Fab segment, which binds to antigens. The Fc region plays a vital role in the body's defense mechanisms.
What are the key functions of the Fc region?
The Fc region facilitates important processes such as opsonization, complement activation, and antibody-dependent cellular cytotoxicity (ADCC), which are essential for immune defense.
How do therapeutic antibodies utilize the Fc region?
Therapeutic antibodies, like trastuzumab, use the Fc region to interact with immune defense cells, enhancing their ability to target and eliminate cancer cells. Modifications to the Fc region can also improve the pharmacokinetic properties of therapeutic proteins, leading to better treatment efficacy.
What role does the Fc region play in interactions with Immunoglobulin G (IgG)?
The Fc region interacts strongly with IgG antibodies, allowing them to attach to Fc receptors on defense cells such as macrophages and natural killer (NK) cells. This interaction is crucial for initiating immune responses like phagocytosis and ADCC.
How do modifications to the Fc region affect therapeutic outcomes?
Modifications to the Fc region can enhance interactions with IgG and serum albumin, leading to improved treatment outcomes. For instance, long-acting biologics use these interactions to extend their half-lives in circulation.
What is the significance of the Fc region's interaction with serum albumin?
The binding of the Fc region to serum albumin stabilizes medical proteins and significantly reduces their clearance rates, making it important in the design of effective biologics.
What role does the neonatal Fc receptor (FcRn) play in relation to the Fc region?
The neonatal Fc receptor (FcRn) helps recycle IgG and protects it from degradation, which is essential for maintaining IgG homeostasis in the body.
Why is understanding the Fc region important for therapeutic molecule design?
Understanding the composition and function of the Fc region is fundamental for developing effective therapeutic proteins, as it directly influences their interactions with immune cells and overall clinical effectiveness.