


In the complex realm of clinical research, the significance of data archiving is paramount, especially within Bulgaria's regulatory framework. Research directors face the challenge of navigating a labyrinth of rules designed to protect participant confidentiality and ensure adherence to rigorous legal standards. This article explores ten crucial trial data archiving rules that empower research leaders to boost operational efficiency and maintain the integrity of their studies. As the stakes escalate, organizations must ask themselves: how can they not only achieve compliance but also position themselves for success in an increasingly competitive Medtech landscape?
bioaccess® offers vital information archiving services essential for maintaining the integrity and availability of clinical study records across Latin America. By leveraging advanced technologies and established best practices, bioaccess® ensures that all experimental data is securely archived, compliant with regulatory standards, and readily retrievable when needed. This capability not only accelerates the testing process but also enhances the overall quality of research outcomes.
In the rapidly evolving Medtech landscape, bioaccess® stands out by effectively addressing key challenges faced by innovators. With expertise in managing:
bioaccess® expedites studies by achieving patient enrollment rates that are 50% faster. This efficiency translates into significant cost savings, with an estimated $25K saved per patient, making bioaccess® an invaluable partner for Medtech, Biopharma, and Radiopharma innovators.
Collaboration with bioaccess® not only streamlines the research process but also positions organizations for success in a competitive market. As you consider your own challenges in clinical research, think about how partnering with a trusted expert like bioaccess® can enhance your outcomes and drive innovation.
In Bulgaria, adherence to the trial data archiving rules in Bulgaria is paramount for compliance with stringent regulatory frameworks in medical studies. This includes adherence to the General Data Protection Regulation (GDPR) and local privacy laws. Research directors must ensure that all data is archived following the trial data archiving rules in Bulgaria, which not only protects participant confidentiality but also aligns with the retention periods specified by the Bulgarian Personal Data Protection Act.
To maintain compliance and safeguard the integrity of medical investigations, comprehensive management services are essential. These services encompass:
Understanding these regulations is crucial for research directors, as it guarantees that their studies meet all necessary legal requirements.
By grasping the complexities of these frameworks, research directors can navigate the Medtech landscape effectively, addressing key challenges while ensuring the protection of participant data.
Data integrity and security stand as critical pillars in the realm of clinical trials. Bioaccess is dedicated to upholding these essential standards through its comprehensive clinical trial management services. Research directors must prioritize the implementation of robust information management systems, incorporating:
to safeguard sensitive information. Have you considered how electronic data capture (EDC) systems can enhance accuracy and reduce the risk of errors? With Bioaccess's expertise in:
directors can confidently focus on maintaining information integrity and security. This commitment ensures that their trials yield reliable and trustworthy outcomes.
Creating robust information retention policies is essential for effective research management. These policies must:
In Bulgaria, the trial data archiving rules require that research information be retained for a minimum of 10 years after the conclusion of a study. However, it is crucial to understand that both the sponsor and investigator are obligated to preserve the content of the master file for at least 25 years post-research conclusion, following the trial data archiving rules in Bulgaria. This aligns with the broader European Union Clinical Trials Regulation, which underscores the importance of long-term information integrity and accessibility.
Research leaders should regularly evaluate and update these policies to ensure compliance with evolving regulations and best practices, thereby safeguarding the integrity of their studies. Furthermore, both paper and electronic records must be systematically archived, and organizations must remain vigilant regarding the ongoing challenges of compliance with the General Data Protection Regulation (GDPR). By prioritizing these retention policies, research entities can enhance their operational efficiency and uphold the highest standards of accountability.
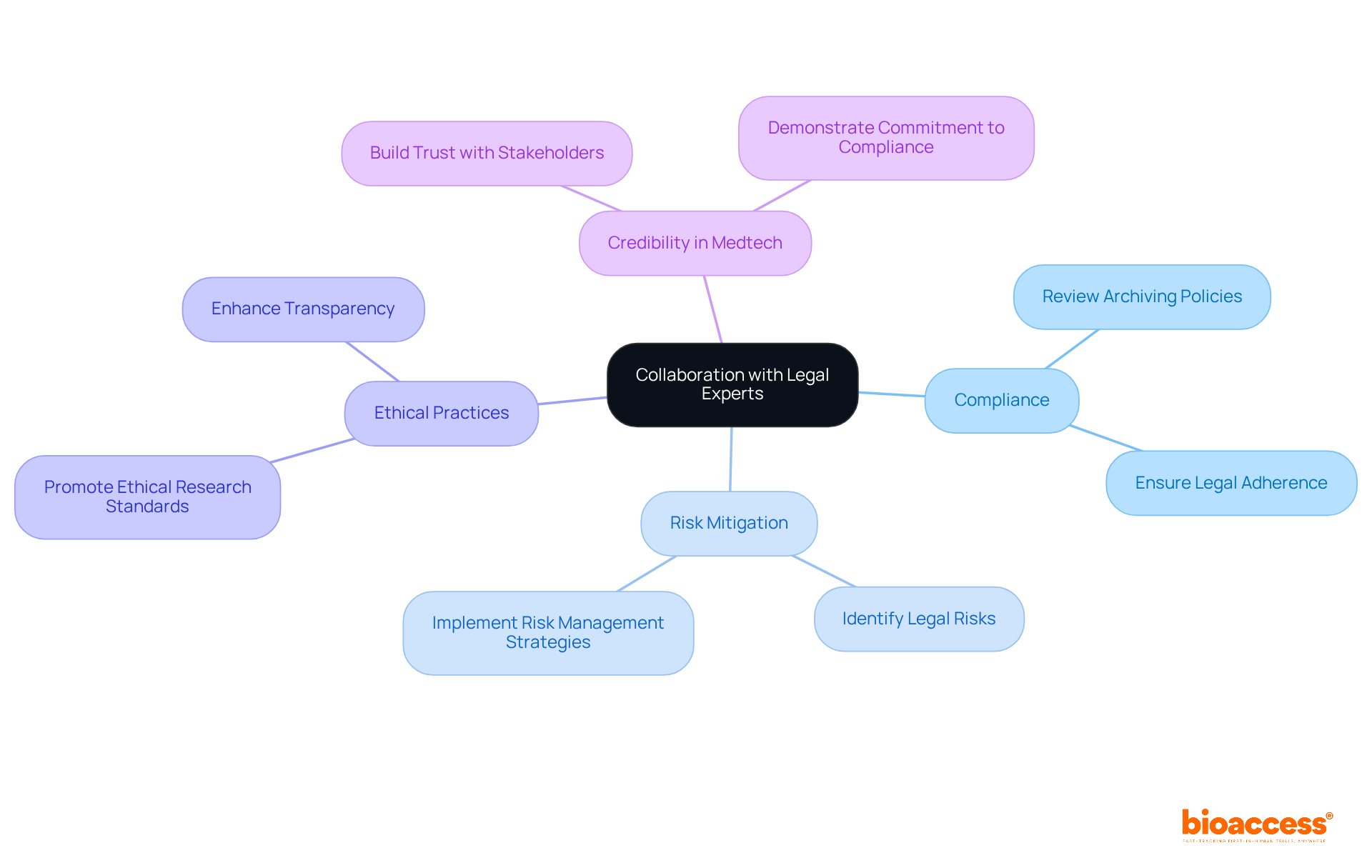
Electronic Data Capture (EDC) systems are essential in modern clinical research, significantly enhancing the efficiency of data collection, management, and storage. By transitioning from traditional paper-based methods, these systems not only minimize data entry errors but also improve accessibility and data integrity. With nearly 80 percent of research studies now utilizing EDC systems, their importance in the industry is undeniable.
Implementing EDC solutions allows project leaders to streamline data archiving processes, ensuring that all research information is securely stored in a centralized digital platform. This setup facilitates easy retrieval for future analysis, ultimately leading to quicker decision-making and better compliance with regulatory standards. Moreover, EDC systems can reduce operational costs by up to 30%, making them a cost-effective choice for managing research data.
As the market for electronic data collection technologies is projected to grow at a compound annual growth rate of 15.8% from 2024 to 2033, the shift towards digital solutions in clinical research is clear. By adopting EDC systems, research directors can enhance their data management capabilities, ensuring their studies are not only efficient but also in line with the latest technological advancements.
In conclusion, the integration of EDC systems is not just a trend; it’s a necessary evolution in clinical research that promises improved efficiency and compliance. Research directors should consider this transition to stay ahead in the rapidly evolving Medtech landscape.
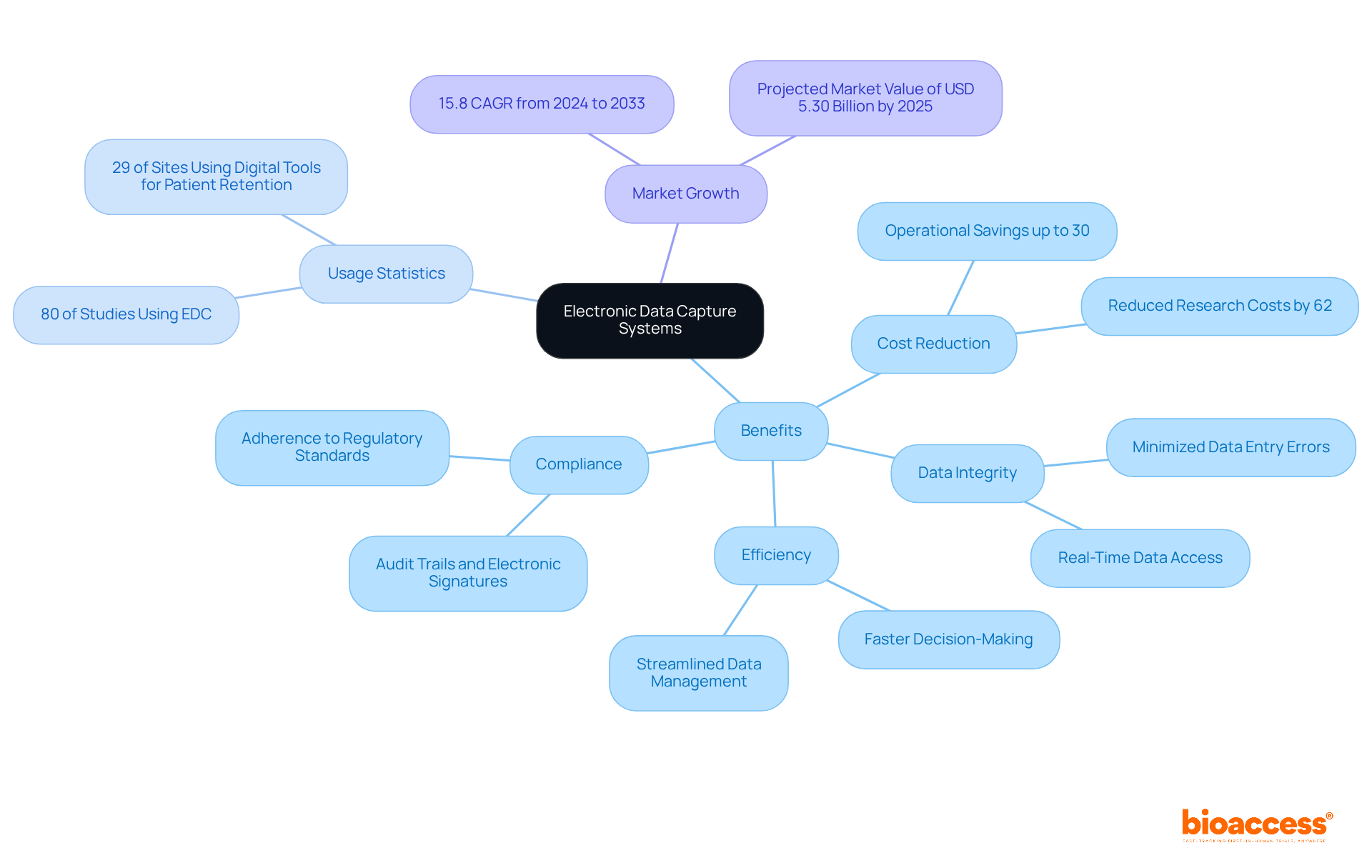
Training personnel on archiving protocols and best practices is crucial for ensuring compliance and maintaining integrity in clinical trials. Research leaders must establish a structured training program that includes regular sessions focused on the latest regulations, archiving technologies, and efficient information management techniques. This training not only equips staff with the necessary skills to perform their roles effectively but also fosters a culture of compliance and accountability within the organization.
By emphasizing continuous learning, organizations can significantly reduce the risk of security breaches - 78% of organizations faced a breach in the past year - and enhance overall operational efficiency. Implementing flexible learning options, such as self-paced modules and interactive content, can further engage staff and improve retention of critical information. Moreover, creating an environment where employees understand their responsibilities in information management and archiving can lead to better compliance with protocols and a stronger governance framework.
As Christopher Latter notes, a well-designed system ensures every document has a designated place, making retrieval swift and intuitive. Following the 3-2-1 principle for storage - maintaining three copies of information on two distinct media types, with one copy off-site - can enhance security and accessibility. This structured approach not only safeguards vital data but also reinforces the organization's commitment to excellence in clinical research.
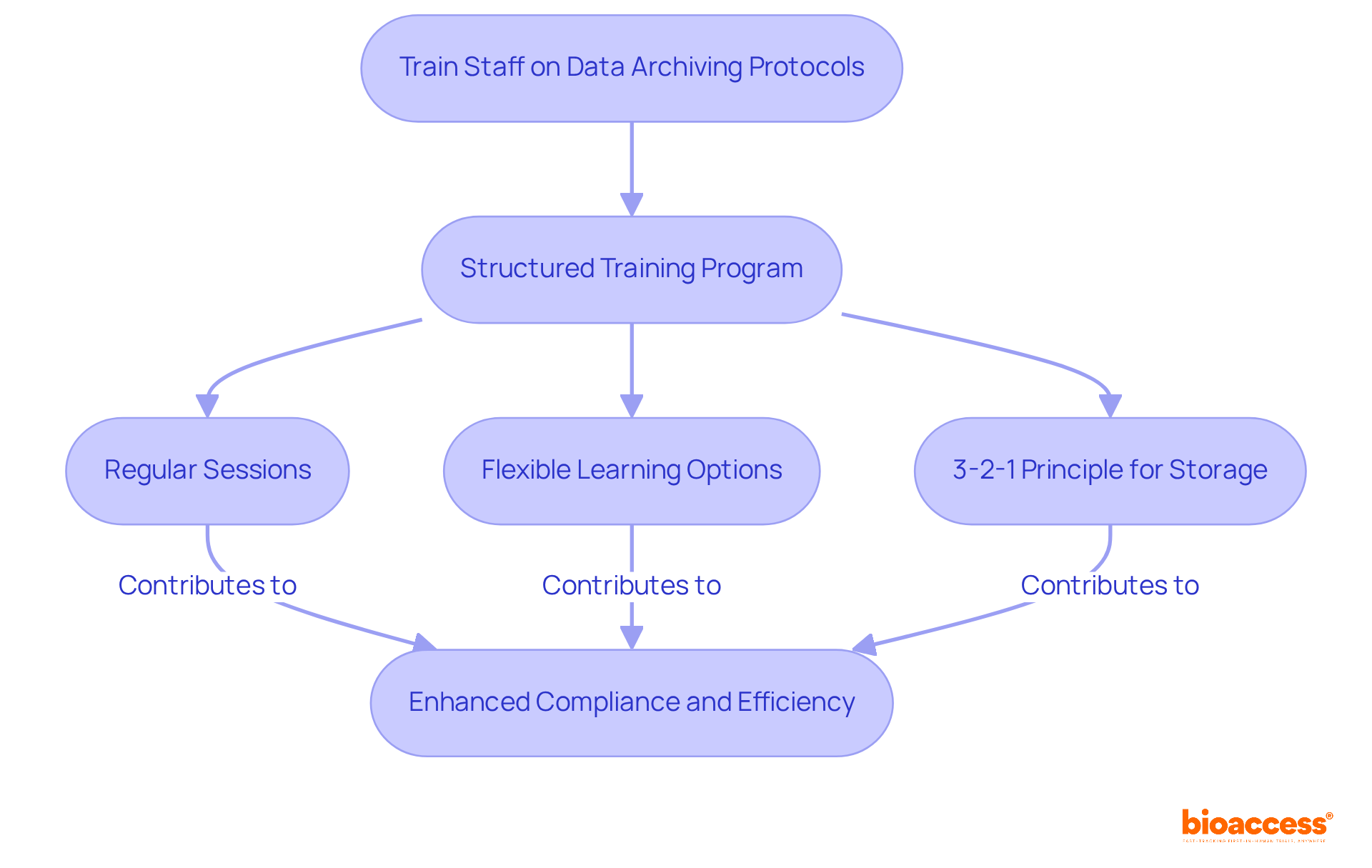
Conducting regular audits of archiving practices is crucial for ensuring compliance with regulatory standards and identifying potential areas for improvement. Research directors must establish a comprehensive audit timetable that includes:
These audits not only help maintain the integrity of information but also prepare the organization for external inspections and evaluations.
By implementing a structured approach to audits, organizations can enhance their operational efficiency and safeguard sensitive data. This proactive measure not only mitigates risks but also fosters a culture of accountability among staff. As the Medtech landscape evolves, staying ahead of compliance requirements becomes increasingly vital.
In conclusion, regular audits are not just a regulatory necessity; they are a strategic advantage that positions organizations for success in the competitive clinical research environment. Collaboration and commitment to best practices will pave the way for future advancements.
Creating a robust disaster recovery plan is crucial for safeguarding trial information against unexpected events such as natural disasters, cyberattacks, or system failures. Research directors must develop a comprehensive strategy that includes:
Moreover, it’s vital to outline clear protocols for information recovery, enabling swift restoration in the face of a disaster. By proactively preparing for potential disruptions, organizations can ensure the continuity of their clinical trials and protect the integrity of their information.
Current trends reveal a growing dependence on cloud-based solutions and automated backup systems, which not only streamline the recovery process but also minimize downtime. Best practices advocate for:
Ultimately, a well-structured disaster recovery plan not only mitigates risks but also reinforces stakeholder confidence in the research process.

Collaborating with legal professionals is crucial for navigating the complex landscape of archiving regulations in clinical research. Research directors must engage legal counsel to thoroughly review their archiving policies and practices, ensuring compliance with both local and international laws. This partnership not only mitigates legal risks but also underscores the organization’s dedication to ethical research practices. By prioritizing this collaboration, organizations can confidently address the challenges posed by regulatory requirements and enhance their credibility in the Medtech landscape.
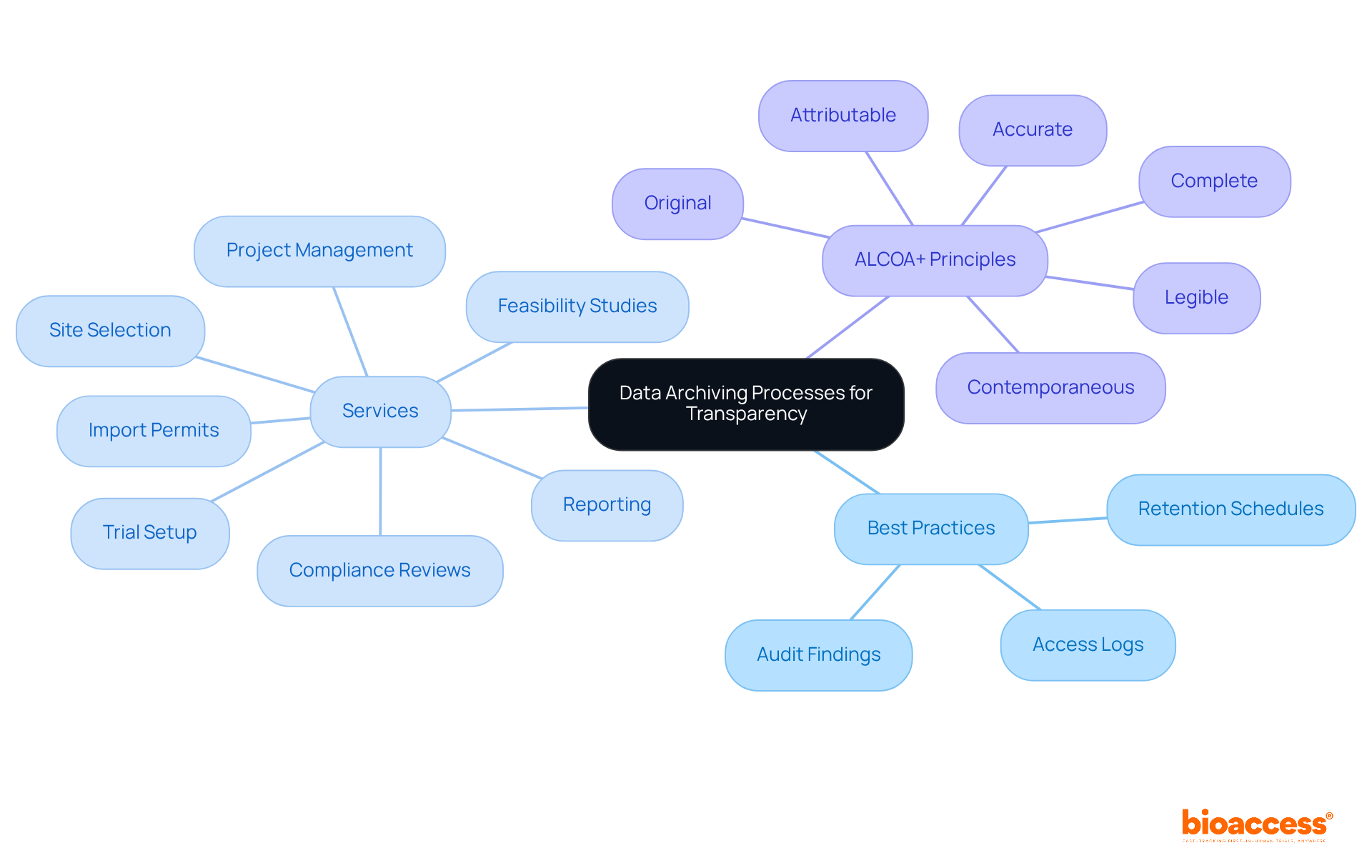
Ensuring openness and responsibility in medical studies hinges on meticulous recording of all information storage procedures. Research directors must implement best practices that involve maintaining detailed records of information management practices, including retention schedules, access logs, and audit findings. This comprehensive documentation not only ensures compliance with regulatory requirements but also establishes a clear trail of accountability, essential for fostering stakeholder trust.
At bioaccess, our extensive clinical trial management services cover:
By adhering to the ALCOA+ principles - Attributable, Legible, Contemporaneous, Original, Accurate, and Complete - we further enhance information integrity. For instance, all source documents must be complete, with no blank fields or missing entries, to avoid ambiguity. Moreover, precise documentation aids in regulatory compliance and ethical review, ultimately enhancing the safety of study participants.
Statistics reveal that over 90% of Preliminary Notices of Noncompliance issued by the FDA have prompted responsible parties to address potential noncompliance, underscoring the importance of maintaining rigorous documentation practices. By ensuring that all data is recorded in real-time and that documentation is readily accessible for audits or inspections, directors can significantly enhance the credibility of their trials and contribute to the advancement of medical knowledge. As the saying goes, "if it wasn’t documented, it didn’t happen," highlighting the critical role of documentation in clinical research.
Understanding and adhering to trial data archiving rules in Bulgaria is essential for research directors who aim to ensure compliance and maintain the integrity of clinical studies. Effective data archiving strategies not only protect participant confidentiality but also streamline research processes, ultimately leading to improved outcomes and success in the competitive Medtech landscape.
Key insights from the article underscore the importance of comprehensive management services, robust data retention policies, and the implementation of electronic data capture systems. Regular training, audits, and collaboration with legal experts are crucial for navigating the complexities of data archiving regulations. Collectively, these practices enhance data integrity, security, and transparency, ensuring that clinical trials meet both local and international standards.
In light of these findings, it is imperative for research directors to prioritize effective data archiving practices. By adopting advanced technologies and following best practices, organizations can safeguard sensitive information, foster accountability, and drive innovation in clinical research. Embracing these strategies positions organizations for compliance and sets the stage for future advancements in the field, ultimately benefiting the broader healthcare community.
What services does bioaccess® provide for clinical trials?
bioaccess® offers vital information archiving services essential for maintaining the integrity and availability of clinical study records, ensuring that all experimental data is securely archived, compliant with regulatory standards, and readily retrievable.
How does bioaccess® enhance the clinical trial process?
bioaccess® accelerates the testing process by achieving patient enrollment rates that are 50% faster, leading to significant cost savings of approximately $25K per patient.
What types of studies does bioaccess® specialize in?
bioaccess® specializes in managing Early-Feasibility Studies, First-In-Human Studies, and other critical experiments.
Why is regulatory compliance important for data archiving in Bulgaria?
Adherence to trial data archiving rules in Bulgaria is crucial for compliance with regulatory frameworks, including the General Data Protection Regulation (GDPR) and local privacy laws, which protect participant confidentiality and ensure alignment with retention periods specified by the Bulgarian Personal Data Protection Act.
What management services are essential for maintaining compliance in clinical trials?
Essential management services include feasibility assessments, site selection, compliance reviews, setup, import permits, project management, and reporting.
How can research directors ensure data integrity and security in clinical trials?
Research directors can ensure data integrity and security by implementing robust information management systems that incorporate encryption, access controls, and regular audits.
What role do electronic data capture (EDC) systems play in clinical trials?
EDC systems enhance accuracy and reduce the risk of errors in clinical trials, contributing to the overall integrity and reliability of the data collected.