


This article delves into the crucial differences between verification and validation within the realm of medical devices. It emphasizes that verification is all about ensuring adherence to specified requirements, while validation confirms that the product truly meets user needs in real-world scenarios. Understanding this distinction is vital for guaranteeing the safety and effectiveness of medical instruments.
The processes involved in both verification and validation are outlined, highlighting their respective roles in product development and regulatory compliance. Verification acts as a checkpoint, ensuring that every requirement is met, while validation serves as a confirmation that the device performs as intended in practical applications. This dual approach not only safeguards patient safety but also enhances the overall reliability of medical devices in the market.
In the ever-evolving Medtech landscape, recognizing these differences is essential for stakeholders aiming to navigate the complexities of clinical research. By fostering collaboration and understanding the significance of both verification and validation, organizations can better address the challenges they face in developing effective medical solutions.
Grasping the distinctions between verification and validation is essential in the medical device sector, where safety and efficacy take precedence. As the industry progresses, the need for clarity in these processes intensifies, providing stakeholders with a clear path to ensure compliance and bolster product reliability. Yet, many find themselves confused by these terms—what exactly differentiates verification from validation, and why is it significant? This article explores the fundamental differences, dispelling common misconceptions and underscoring the critical role both processes play in delivering safe and effective medical solutions.
bioaccess® brings over 20 years of expertise in early-phase clinical studies to enhance the assessment and confirmation methods for medical instruments. By leveraging the dynamic regulatory landscape of Latin America and the diverse patient populations in the Balkans, bioaccess® guarantees that medical products undergo rigorous processes of verification vs validation medical device with remarkable efficiency.
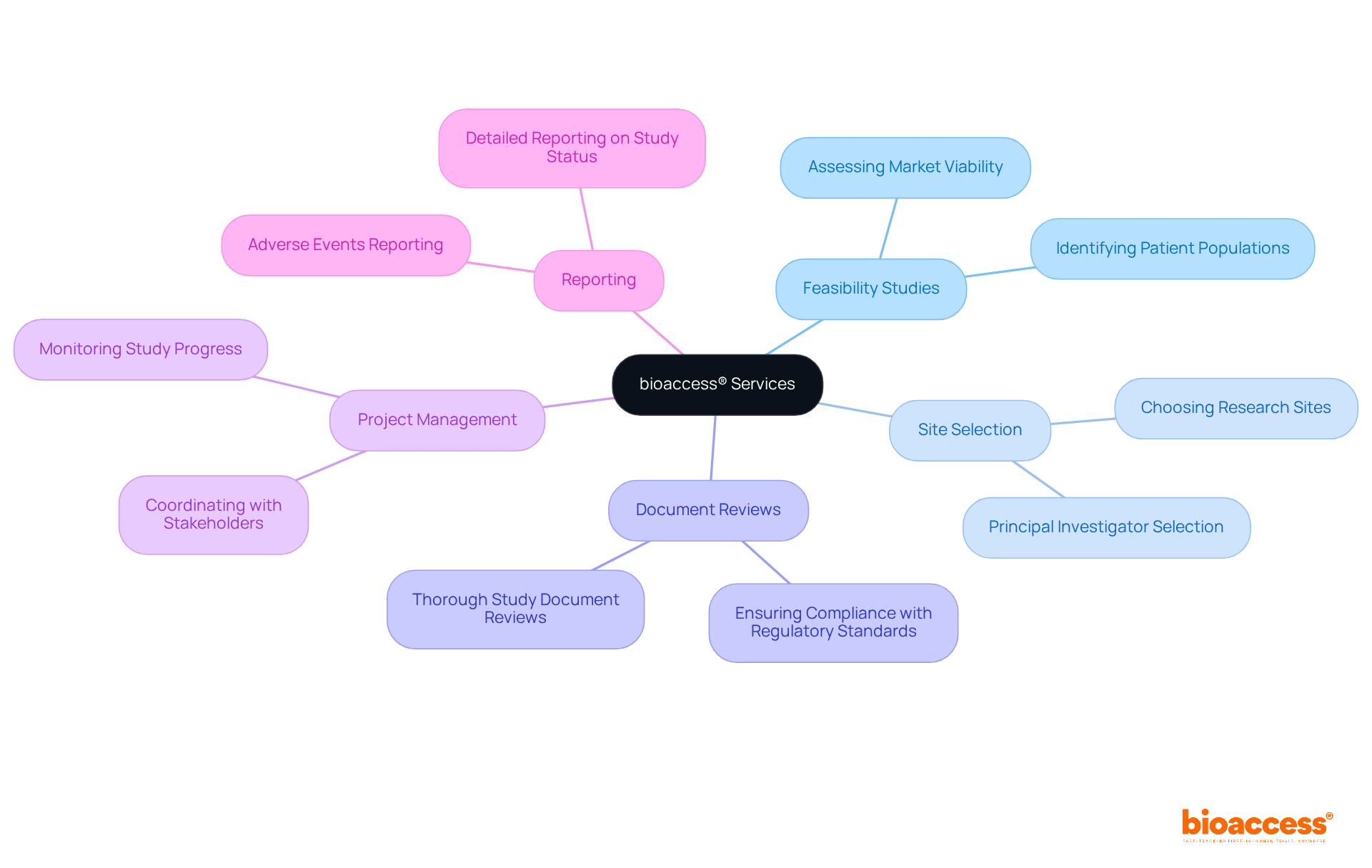
Our extensive services encompass:
This strategic agility not only accelerates market entry but also aligns with international compliance standards, which is vital for innovators eager to introduce their products swiftly and ethically.
The capability to secure ethical approvals in just 4-6 weeks exemplifies how bioaccess® empowers Medtech companies to navigate the complexities of regulatory requirements. This ultimately fosters innovation and enhances patient outcomes. As you consider your own challenges in clinical research, think about how collaboration with bioaccess® can streamline your path to success.
Verification involves assessing whether a product meets specified requirements throughout various development phases. It addresses the question, 'Did we build the product right?' In contrast, validation evaluates whether the product serves its intended purpose in real-world scenarios, answering, 'Did we build the right product?' Understanding the distinctions of verification vs validation medical device is crucial for ensuring that medical instruments are both safe and effective.
In the Medtech landscape, comprehensive clinical trial management services, such as those offered by bioaccess, play a pivotal role in navigating these challenges. These services encompass:
Moreover, bioaccess facilitates the import permits and nationalization of investigational instruments, supported by robust project management and reporting systems that monitor study status and adverse events.
This thorough approach not only aids in the assessment and approval processes but also aligns with the regulatory responsibilities of INVIMA, Colombia's national authority overseeing medical equipment. By collaborating with bioaccess, stakeholders can enhance their clinical research efforts, ensuring that their products are not only compliant but also effective in meeting the needs of healthcare providers and patients alike.

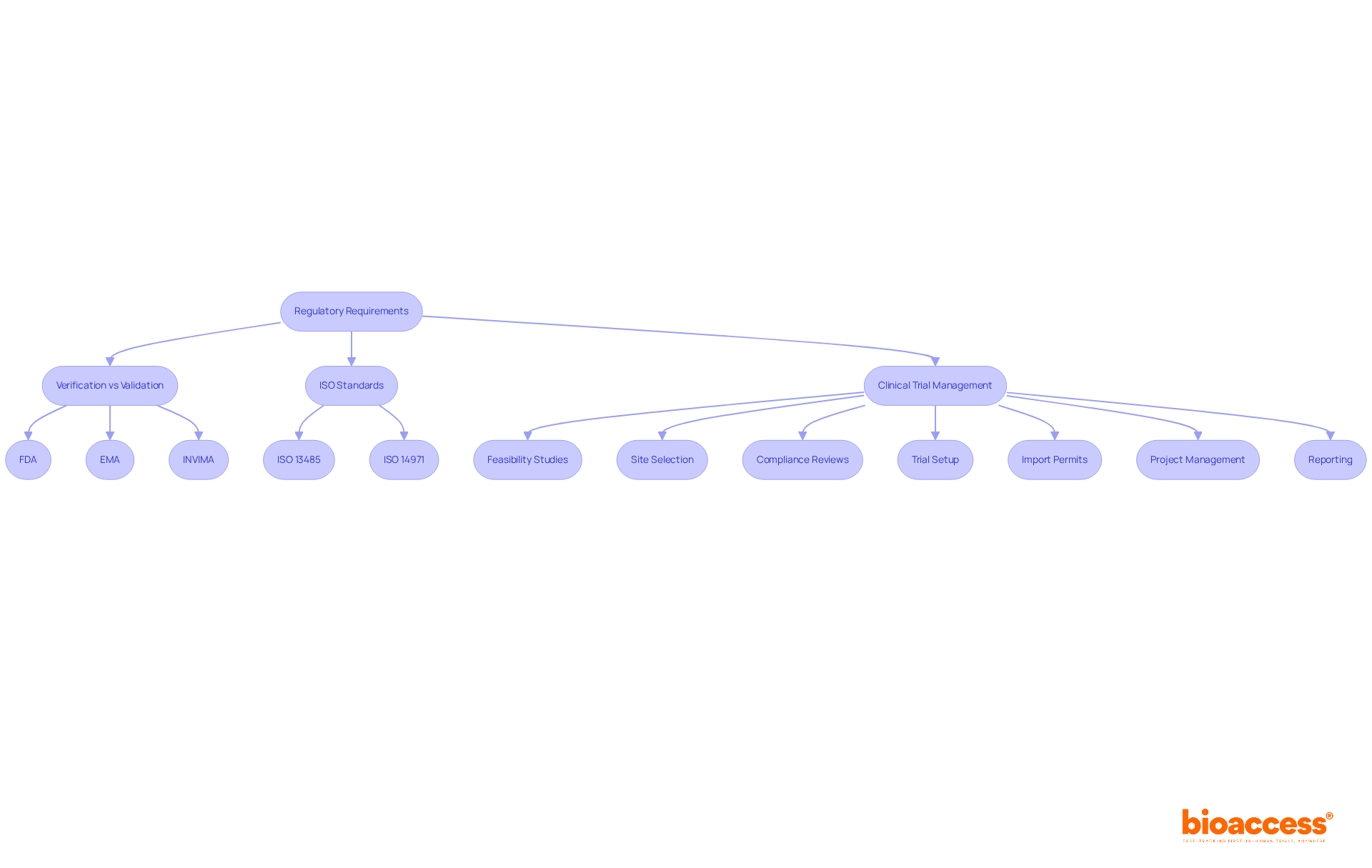
Regulatory authorities such as the FDA and EMA impose stringent processes for verification vs validation medical device for medical instruments. These criteria are essential in the verification vs validation medical device process, ensuring that products are thoroughly evaluated for safety and effectiveness before they reach the market. Adhering to ISO 13485 and ISO 14971 standards is equally crucial, as these frameworks provide a solid foundation for quality management and risk management in medical product development.
In Colombia, INVIMA plays a pivotal role in overseeing medical device regulation, ensuring compliance with national standards. This oversight is vital for maintaining the integrity of the healthcare system. Furthermore, bioaccess offers comprehensive clinical trial management services that include:
These services are indispensable for ensuring adherence to regulatory standards throughout the clinical trial phase.
By collaborating with bioaccess, stakeholders can navigate the complexities of clinical research more effectively. The combination of regulatory expertise and clinical trial management not only streamlines processes but also enhances the likelihood of successful product development. As the Medtech landscape continues to evolve, the importance of such partnerships cannot be overstated. Stakeholders are encouraged to consider how these services can address their specific challenges in clinical research.
A common misconception is that checking and confirming are synonymous; however, they serve different purposes. Additionally, many believe that the verification vs validation medical device process is only required at the end of development. In reality, it should be integrated at multiple stages to ensure ongoing compliance. Furthermore, some may think that the concept of verification vs validation medical device is unnecessary, yet it is crucial for confirming that the equipment meets user requirements and intended applications.

In clinical research, the verification vs validation medical device processes are critical for ensuring product efficacy and safety through assessment and confirmation. Assessment may involve rigorous testing of a product's software to address the verification vs validation medical device compliance with design specifications. In contrast, verification vs validation medical device often includes clinical trials aimed at validating the product's performance within a patient population. For instance, a new cardiac monitor undergoes verification vs validation medical device processes, beginning with bench testing for assessment and subsequently confirmed through trials involving real patients, allowing for evaluation under practical conditions.
bioaccess™ plays a pivotal role in facilitating first-in-human clinical studies, exemplified by Avantec Vascular's groundbreaking vascular trial in Latin America. By assisting in the selection of a principal investigator and the submission of the regulatory dossier, bioaccess™ ensures that both verification vs validation medical device processes are meticulously followed. This adherence is essential for achieving regulatory compliance and successful outcomes in clinical research.
The Medtech landscape is evolving, and the challenges faced in clinical trials are significant. Collaboration among stakeholders is vital to navigate these complexities effectively. As we look to the future, the importance of strategic partnerships and innovative solutions cannot be overstated. By leveraging expertise and resources, we can enhance the efficiency and effectiveness of clinical research, ultimately leading to better patient outcomes.

In the context of medical device development, understanding the difference between verification vs validation medical device is crucial, as both involve distinct methodologies to ensure product quality and compliance. Verification typically encompasses inspections, analyses, and testing against predefined specifications, focusing on whether the product meets established requirements. For example, validation techniques may involve statistical sampling plans that determine sample sizes and acceptance criteria, ensuring that individual units conform to quality standards. These plans are vital for design verification (STAT-04) and process confirmation (STAT-03), providing a statistical foundation for confirming product reliability.
Conversely, validation emphasizes user testing, clinical trials, and real-world assessments to confirm that the product meets user needs and intended uses. This phase is essential for understanding how the device performs in practical scenarios, often leading to insights that can refine product design. Clinical trials conducted by bioaccess, including Early-Feasibility Studies (EFS) and First-In-Human Studies (FIH), may reveal user preferences and usability challenges that were not apparent during validation.
Industry leaders highlight the distinctions between verification vs validation medical device, underscoring the significance of both methods. While verification ensures that the product is constructed correctly, validation confirms that the appropriate product is designed for the target audience. This comprehensive approach to testing throughout the development lifecycle is crucial for achieving regulatory compliance and ensuring patient safety.
Incorporating checks and assessments not only enhances product quality but also accelerates the route to market, enabling innovators to deliver effective medical solutions to healthcare providers and patients more efficiently. Case studies exploring the use of sampling plans in design assessment and procedure confirmation illustrate the practical implications of these methodologies, emphasizing their importance in the medical device sector.

Design assessment is crucial in ensuring that design outputs align with specified design inputs, while verification vs validation medical device confirms that the final product meets user needs and intended applications. These processes are essential in product development, facilitating early identification of potential issues and significantly reducing the risk of costly recalls or redesigns later on. For instance, effective design assessment can lead to a 40% reduction in protocol modifications during trials, underscoring its role in enhancing operational efficiency.
Moreover, industry specialists emphasize that early problem detection through design assessment not only simplifies development but also enhances the overall quality and safety of medical equipment. By rigorously implementing these methods, manufacturers can ensure their products adhere to regulatory standards and meet user expectations, which is an essential aspect of verification vs validation medical device, ultimately resulting in safer and more effective medical solutions.
At bioaccess®, we leverage our extensive knowledge in overseeing thorough clinical trial services throughout Latin America, including:
Our expertise assists manufacturers in navigating the complexities of design confirmation and assessment, ensuring that their medical products are developed efficiently and effectively.

Thorough documentation is crucial in both confirmation and assessment processes for medical devices. It involves meticulous records of testing protocols, results, and any deviations from expected outcomes. Effective traceability ensures that all requirements are systematically linked to their corresponding assessment and confirmation activities, creating a clear audit trail essential for regulatory compliance and quality assurance. Statistics reveal that organizations with robust documentation practices experience significantly higher rates of regulatory compliance, underscoring the necessity of maintaining comprehensive records.
Experts assert that thorough documentation not only facilitates compliance but also enhances operational efficiency, ultimately leading to improved patient safety and product reliability. As Dr. Frans van der Horst, a Clinical Chemist, states, "We have an obligation to our patients, colleagues, and external clients to provide fast, high-quality services at the lowest cost," emphasizing the critical role of documentation in achieving these objectives. Furthermore, Yolanda van Leusden, Manager of the Department of Chromatography, notes, "One of the main reasons for looking into automation was to cut the costs of testing," which highlights the efficiency gained through meticulous documentation and traceability.
At bioaccess, our comprehensive clinical trial management services encompass:
All these elements rely heavily on thorough documentation to ensure adherence to regulatory standards, including those set by INVIMA, the Colombian National Food and Drug Surveillance Institute.

Insufficient verification vs validation medical device and assessment can lead to severe outcomes, including product recalls, regulatory penalties, and compromised patient safety. A striking example is the recalls of artificial intelligence-enabled medical instruments (AIMIs), where 43.4% occurred within the first 12 months of approval. This statistic underscores the urgent need for efficient verification procedures. Among 950 active implantable medical devices (AIMDs), 60 items were linked to 182 recall events, with diagnostic errors accounting for a significant portion of these incidents. Notably, devices lacking clinical approval experienced an average of 3.4 recalls per device, compared to 1.9 for those that underwent retrospective assessment. This stark contrast highlights the essential requirement for thorough verification vs validation medical device procedures to prevent unexpected failures that could jeopardize patient safety and tarnish manufacturers' reputations.
Expert opinions suggest that the correlation between public company status and elevated recall rates may reflect pressures for expedited launches. This further emphasizes the necessity for enhanced premarket clinical testing and robust postmarket surveillance measures. Ultimately, ensuring comprehensive assessment and confirmation is crucial to mitigate risks and safeguard patient safety.

To understand verification vs validation in medical device context, it's essential to recognize that:
Both processes are crucial for ensuring the safety and effectiveness of medical equipment.
In the competitive Medtech landscape, bioaccess® offers expert services that enable startups in Medtech, Biopharma, and Radiopharma to accelerate their clinical trials, achieving regulatory approval and trial data 40% faster.
Proper documentation, strict adherence to regulatory requirements, and a clear understanding of the consequences of inadequate practices are vital for successful device development, particularly when aiming to progress to the next phase of clinical studies.

Understanding the nuances between verification and validation in medical devices is critical for ensuring product safety and effectiveness. Verification confirms that a product is built according to specified requirements, while validation assesses whether the product meets user needs in real-world applications. This distinction is essential for stakeholders in the Medtech industry, as it directly impacts the quality and reliability of medical instruments.
Throughout this discussion, we’ve explored key points, including:
We also highlighted common misconceptions surrounding these processes and the potential consequences of inadequate verification and validation, such as product recalls and compromised patient safety. By leveraging the services offered by bioaccess®, companies can navigate the complexities of regulatory requirements and enhance their clinical research efforts.
Ultimately, prioritizing robust verification and validation practices is not just a regulatory necessity; it’s a commitment to patient safety and product integrity. Stakeholders are encouraged to embrace these processes as integral components of medical device development, fostering innovation while ensuring that products meet the highest standards of quality and efficacy. Collaborating with experienced partners like bioaccess® can significantly streamline this journey, paving the way for successful outcomes in the ever-evolving landscape of medical technology.
What is bioaccess® and its expertise in the medical device industry?
bioaccess® brings over 20 years of expertise in early-phase clinical studies, enhancing assessment and confirmation methods for medical instruments, particularly in the dynamic regulatory landscape of Latin America and the Balkans.
What services does bioaccess® offer?
bioaccess® offers a range of services including feasibility studies, selection of research sites and principal investigators, thorough reviews of study documents, project management and monitoring, and detailed reporting on study status and adverse events to ensure regulatory compliance.
How does bioaccess® facilitate market entry for medical devices?
bioaccess® accelerates market entry by securing ethical approvals in just 4-6 weeks and aligning with international compliance standards, which is essential for innovators looking to introduce their products quickly and ethically.
What is the difference between verification and validation in medical devices?
Verification assesses whether a product meets specified requirements throughout development (i.e., "Did we build the product right?"), while validation evaluates whether the product serves its intended purpose in real-world scenarios (i.e., "Did we build the right product?").
Why are regulatory requirements important in the verification and validation process?
Regulatory authorities like the FDA and EMA impose stringent processes to ensure that medical instruments are thoroughly evaluated for safety and effectiveness before market release. Adhering to ISO 13485 and ISO 14971 standards is also crucial for quality and risk management.
What role does INVIMA play in medical device regulation in Colombia?
INVIMA oversees medical device regulation in Colombia, ensuring compliance with national standards, which is vital for maintaining the integrity of the healthcare system.
How can stakeholders benefit from collaborating with bioaccess®?
By collaborating with bioaccess®, stakeholders can enhance their clinical research efforts, navigate regulatory complexities more effectively, and increase the likelihood of successful product development through comprehensive clinical trial management services.