


This article delves into the key differences between Phase 2a and Phase 2b clinical trials, underscoring their distinct objectives, participant sizes, regulatory requirements, endpoints, and implications for stakeholders. Understanding these differences is crucial for anyone involved in clinical research.
Phase 2a trials primarily focus on evaluating safety and initial efficacy within smaller groups. In contrast, Phase 2b trials engage larger cohorts to optimize dosages and assess comprehensive treatment effectiveness. This distinction is vital for advancing to later stages of clinical research, where the stakes are higher and the need for robust data is paramount.
As we navigate the complexities of the Medtech landscape, it becomes evident that collaboration is essential. By addressing the challenges inherent in clinical trials, stakeholders can better position themselves for success.
In summary, recognizing the nuances between Phase 2a and Phase 2b trials not only enhances our understanding of clinical research but also emphasizes the importance of strategic collaboration moving forward.
Understanding the nuances between Phase 2a and Phase 2b clinical trials is essential for stakeholders navigating the dynamic landscape of medical research. These two stages, while interconnected, serve distinct purposes that can significantly influence the trajectory of drug development. As the demand for efficient and effective clinical trials continues to rise, exploring these differences not only highlights the potential for improved patient outcomes but also reveals strategic advantages that innovative companies can leverage.
What challenges and opportunities emerge when navigating the complexities of these phases? How can stakeholders harness this knowledge to enhance their research strategies?
bioaccess® is revolutionizing clinical trials phase 2a 2b, particularly by leveraging Colombia's strategic advantages. With cost savings exceeding 30% compared to North America and Western Europe, bioaccess® empowers Medtech startups to conduct trials with remarkable efficiency. The regulatory landscape in Colombia allows for ethical approvals in just 4-6 weeks—significantly faster than traditional markets. Moreover, recruitment benefits from a population of over 50 million, with 95% covered by universal healthcare.
This global-first medical agility not only accelerates enrollment—achieving speeds 50% quicker than in other regions—but also guarantees high-quality data collection, bolstered by Colombia's top-ranked healthcare system. By connecting innovative firms with diverse patient groups, bioaccess® ensures that clinical studies are both efficient and effective, driving forward medical advancements.
In a landscape where time and quality are paramount, bioaccess® stands out as a beacon of opportunity for Medtech startups. The collaboration between innovative companies and a robust patient base is essential for overcoming the challenges of clinical research. As the Medtech sector continues to evolve, partnering with bioaccess® could be the key to unlocking new possibilities in clinical trials phase 2a 2b.

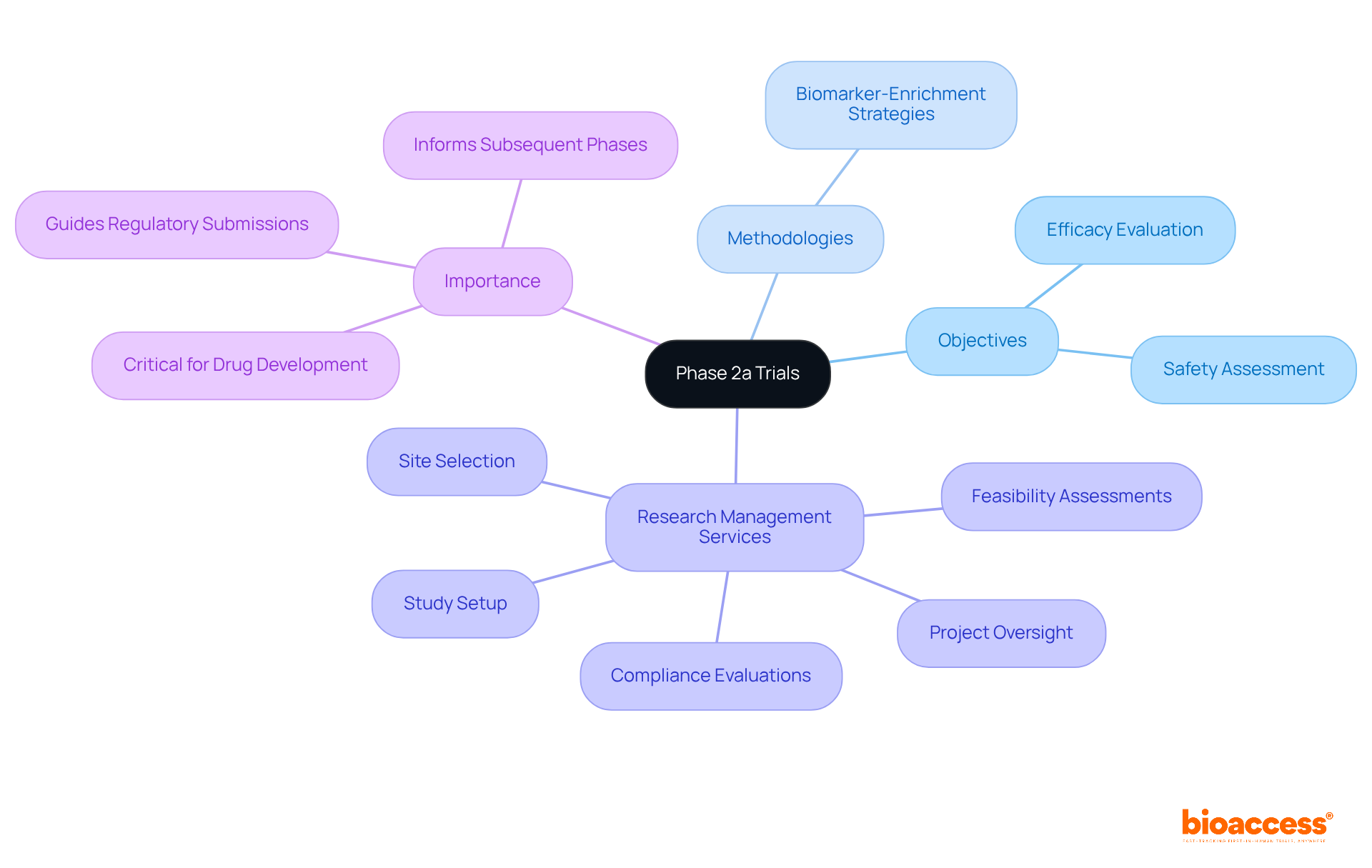
Phase 2a studies play a pivotal role in assessing the effectiveness and safety of treatments within a limited participant group, typically ranging from 20 to 100 individuals. Recent findings from 2025 underscore the importance of these trials in evaluating a treatment's potential, particularly in identifying adverse effects and initial efficacy. For example, the Phase 2a study of obefazimod revealed a promising safety profile, with 67% of participants experiencing significant clinical responses, highlighting the treatment's potential for chronic inflammatory diseases.
Current trends indicate that Phase 2a studies are increasingly incorporating innovative methodologies, such as biomarker-enrichment strategies, which enhance patient stratification and improve success rates. Clinical researchers emphasize that these clinical trials phase 2a 2b are essential for determining whether a treatment should progress to Phase 2b, where larger cohorts are analyzed. The insights gained from Phase 2a studies not only shape the design of subsequent phases but also provide critical information for regulatory submissions.
At bioaccess, we understand the complexities involved in conducting Phase 2a studies. Our comprehensive research study management services encompass:
We prioritize meticulous reporting processes to monitor study status, inventory, and adverse events, which are vital for informed decision-making. Experts in the field advocate for a thorough evaluation of both effectiveness and safety during Phase 2a assessments, as these analyses are crucial for mitigating risks in later development stages. The ongoing evolution of study designs reflects a commitment to improving outcomes and ensuring that only the most promising therapies advance through the clinical pipeline. Notably, the overall likelihood of success for Phase 2 assessments is recognized to be at least 80%, underscoring their significance in the drug development landscape.
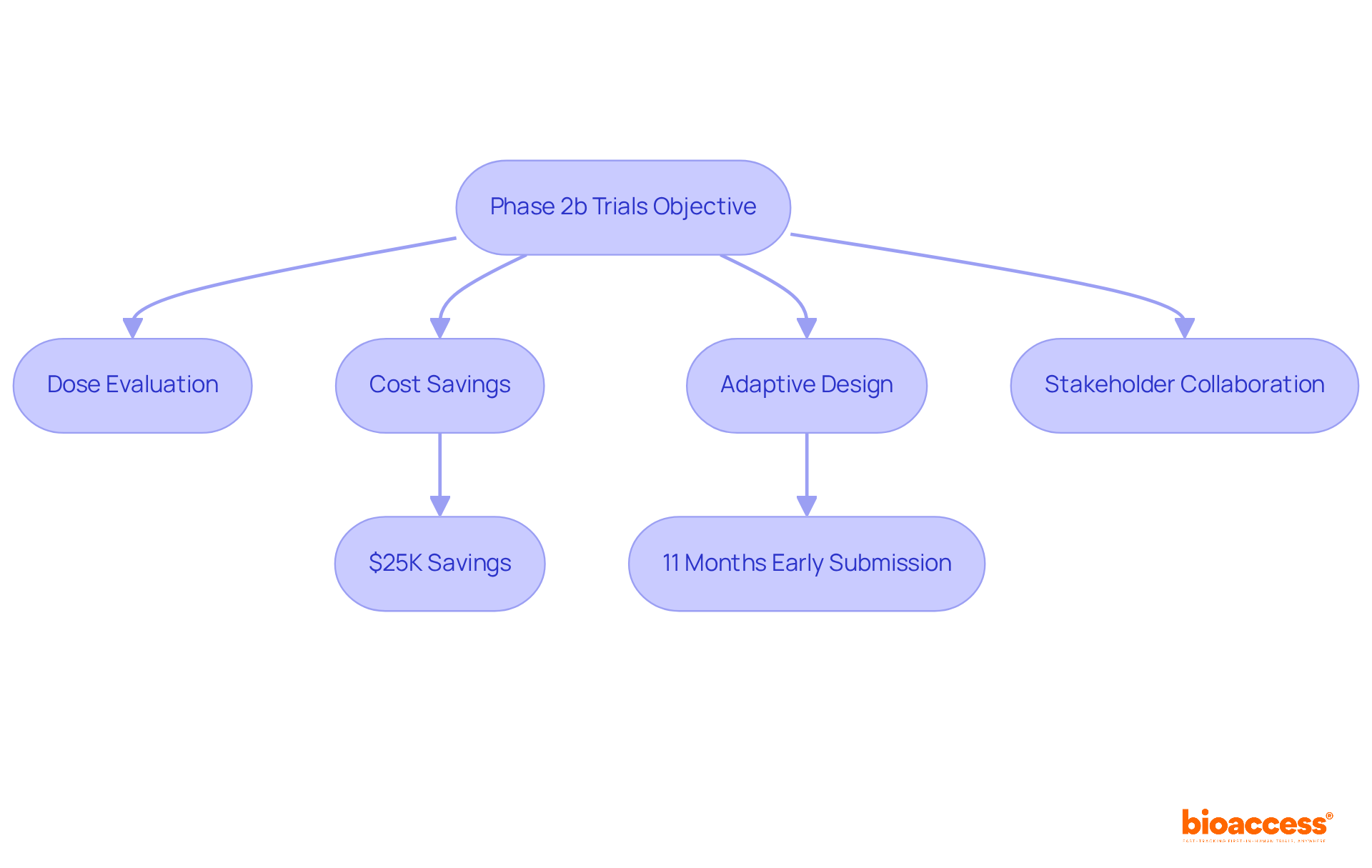
Assessments in clinical trials phase 2a 2b play a crucial role in clinical research, aiming to thoroughly evaluate the effectiveness of treatments by exploring various dosages and their impacts. This phase of clinical trials phase 2a 2b is essential for optimizing treatment regimens, ensuring that the most effective dose is identified while maintaining a strong focus on safety. Notably, the cost-to-speed ratio achieved by bioaccess® is remarkable, with savings of $25K for individuals and the ability to submit PMA data a staggering 11 months earlier than anticipated. Such efficiencies are vital for designing Stage 3 studies, where larger participant groups are examined, particularly in overcoming recruitment challenges that often hinder early-stage clinical trials.
Moreover, the integration of adaptive designs in clinical trials phase 2a 2b studies allows for real-time adjustments based on interim data, significantly enhancing study efficiency and responsiveness. This adaptability not only streamlines the research process but also addresses the pressing need for timely and effective clinical solutions. As we move forward, collaboration among stakeholders will be key to navigating the complexities of clinical research and ensuring successful outcomes.
Clinical trials phase 2a 2b typically involve a focused group of 50 to 200 participants in stage 2a studies. This targeted approach is vital for a thorough evaluation of safety and dosage, which is essential for understanding how treatments interact with the human body. In contrast, clinical trials phase 2a 2b studies expand the cohort size to between 100 and 300 participants, facilitating a more comprehensive assessment of the treatment's effectiveness across diverse demographics. This larger scale is crucial for capturing variability in responses, as approximately 33% of medications in Phase 2b progress to the next stage of development.
The success of these studies hinges on meticulous design and participant recruitment strategies, which can consume 30-40% of the total budget. bioaccess® stands out in this arena, connecting innovative Medtech, Biopharma, and Radiopharma startups with top-tier research facilities, ensuring effective participant recruitment and regulatory approval processes. For instance, the BMT CTN 0601 study highlights the importance of rigorous oversight in clinical trials phase 2a 2b, where monitoring potential adverse events is critical for participant safety.
As clinical researchers emphasize, "The insights obtained from these studies can result not only in regulatory approval but also in progress in medical science that offers individuals new options." It's also noteworthy that around 15% of patients in various studies may experience severe negative events, underscoring the necessity for robust monitoring protocols. Understanding the differences in group sizes between clinical trials phase 2a 2b is essential for stakeholders aiming to enhance research strategies and improve the likelihood of favorable outcomes.
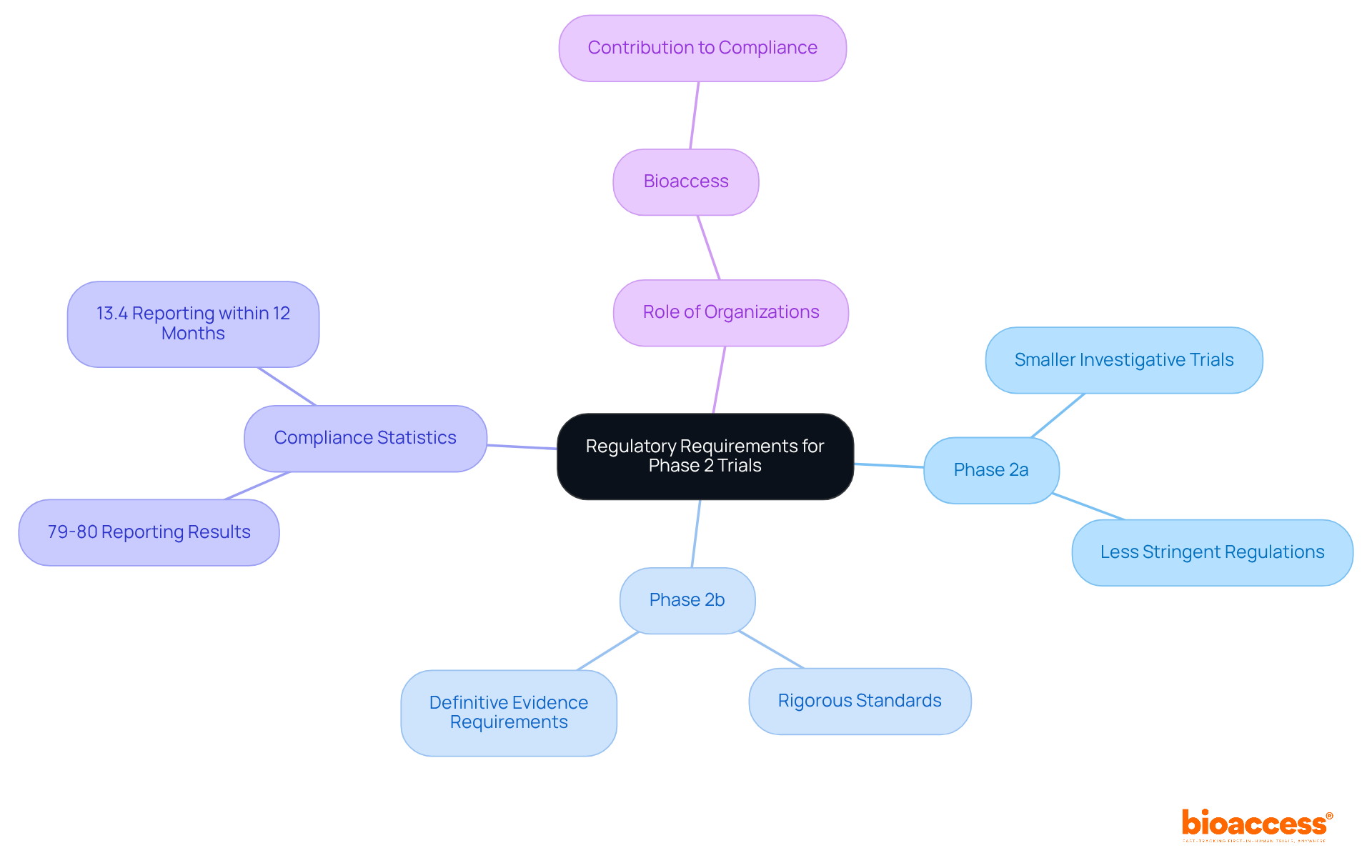
Regulatory requirements for clinical trials phase 2a 2b differ significantly, reflecting the distinct objectives of each stage. Stage 2a studies, which are part of clinical trials phase 2a 2b, being smaller and investigative, often encounter less stringent regulations. In contrast, clinical trials phase 2a 2b are subject to more rigorous standards, as they aim to provide definitive evidence of a drug's efficacy and safety across larger patient populations.
Statistics indicate that compliance rates for industry-funded studies have improved, with approximately 79-80% reporting results or having acceptable reasons for delays. However, only 13.4% of studies reported results within the required 12-month window after completion, highlighting the challenges in meeting regulatory expectations. Navigating these complex regulations is essential for the successful execution of tests.
Organizations like bioaccess play a crucial role in ensuring compliance, utilizing their expertise to assist sponsors through the evolving regulatory landscape. Their involvement not only streamlines the process but also enhances the overall integrity of clinical research.
In clinical trials phase 2a 2b studies, key endpoints encompass initial efficacy metrics, safety evaluations, and pharmacokinetic information, all of which are crucial for assessing a treatment's potential. These metrics guide researchers in determining whether a treatment is effective and safe enough to progress to the next phase. Commonly utilized endpoints include changes in biomarkers, patient-reported outcomes, and adverse event rates.
For instance, pharmacokinetic data gathering in Stage 2a studies has shown that effective monitoring can lead to a 20-30% reduction in adverse effects. Recent research involving Lomecel-B™ revealed a statistically significant enhancement in brain volume loss among Alzheimer's patients. Looking ahead to 2025, essential metrics for success in clinical trials phase 2a 2b include achieving a progression-free survival (PFS) rate of at least 85% while ensuring that safety evaluations report no significant adverse events.
To support these experiments, bioaccess offers extensive clinical study management services, including:
These services are vital for ensuring that examinations are conducted efficiently and in accordance with regulatory standards, ultimately influencing local economies through job creation and healthcare enhancement.
Clinical researchers emphasize the importance of these metrics, noting that favorable results in clinical trials phase 2a 2b can attract additional funding and facilitate smoother transitions to subsequent studies. As Dr. Anne Visbecq, Chief Medical Officer, stated, "These findings confirm the hypotheses raised from preclinical studies and strongly support the premise of moving forward with pivotal studies." Therefore, assessing success in Stage 2a evaluations necessitates a multifaceted approach, integrating pharmacokinetic data, safety assessments, and patient outcomes to inform future drug development strategies.
In clinical trials phase 2a 2b, studies play a pivotal role in evaluating treatment effectiveness, employing a range of comprehensive endpoints. Primary endpoints typically encompass overall response rate (ORR), progression-free survival (PFS), and quality of life measures. Together, these elements form a solid framework for assessing the impact of treatments. For instance, recent findings from clinical trials phase 2a 2b of GH001 for treatment-resistant depression revealed a significant 15.5-point placebo-adjusted reduction on the Montgomery-Åsberg Depression Rating Scale (MADRS) by Day 8, alongside a remission rate of 57.5% compared to 0% in the placebo group. This underscores the essential function of ORR in evaluating treatment viability.
Secondary endpoints further enhance the analysis, providing valuable insights into adverse events and patient-reported outcomes (PROs). Research specialists emphasize that the careful selection of these endpoints is vital for accurately gauging a treatment's potential prior to clinical trials phase 2a 2b and advancing to larger Stage 3 studies. The data gathered during this phase not only informs regulatory decisions but also influences future research trajectories, establishing it as a cornerstone of the clinical development process.
Clinical trials phase 2a 2b studies typically have a shorter duration, ranging from several months to a year, influenced by factors such as treatment type and the effectiveness of patient recruitment. In contrast, clinical trials phase 2a 2b studies generally extend from one to two years, necessitating more thorough data collection and evaluation. This longer timeline significantly affects resource allocation and planning for subsequent phases. Notably, the average duration for clinical trials phase 2a 2b studies has increased from 37 months during 2011-2015 to 41 months in 2016-2021, reflecting the growing complexity of study designs and the need for rigorous data oversight.
Comprehensive research management services, like those offered by bioaccess, are crucial in navigating these complexities. These services include:
They ensure that the right research locations and principal investigators are chosen to optimize patient recruitment and retention. Alarmingly, recent trends show that up to 80% of research studies fail to meet their timelines, underscoring the importance of effective planning and resource management.
Insights from medical researchers emphasize that improving resource distribution in clinical trials phase 2a 2b is vital for maintaining study integrity and achieving successful outcomes. Additionally, the increasing demand for study participants and the complexity of protocols present significant challenges in recruitment and retention. Therefore, it is essential for clinical research planning to address these critical aspects.

Financial support for clinical trials phase 2a 2b studies typically remains limited, reflecting their exploratory nature and smaller participant groups, which range from 50 to 200 individuals. In contrast, clinical trials phase 2a 2b studies demand a more substantial financial commitment due to their focus on extensive data collection and larger patient cohorts, often involving hundreds of participants. The overall costs for clinical trials phase 2a 2b can fluctuate between $7 million and $20 million, while the expenses for clinical trials phase 2b can escalate significantly, requiring careful budget planning and resource allocation. Organizations must strategically evaluate their financial frameworks to ensure both phases receive the necessary backing.
This is where bioaccess excels, offering cost-effective solutions that enhance research efficiency and effectiveness. By leveraging insights from industry leaders and real-world case studies, bioaccess aids clients in navigating the complexities of budget allocation, ensuring that funding aligns with the specific needs of each project phase. As you consider your own challenges in clinical research, think about how strategic financial support can make a difference in your studies.
The differences between clinical trials phase 2a and 2b studies carry significant implications for clinical research participants, including sponsors, regulatory authorities, and research institutions. Understanding these distinctions is essential for effective strategic planning, resource allocation, and compliance with regulatory standards. For instance, clinical trials phase 2a and 2b typically involve smaller groups of 50 to 200 individuals, focusing on safety and optimal dosage evaluation. In contrast, clinical trials phase 2a and 2b expand participant criteria to evaluate treatment effectiveness across a broader demographic, providing crucial evidence for progression to Stage 3 research.
Statistics reveal that approximately 32.5% of individuals in clinical trials phase 2a and 2b studies advance to Stage 3 assessments, underscoring the importance of positive outcomes in these clinical trials. Additionally, participant recruitment can account for 30-40% of Stage 2 budgets, highlighting the need for strategic planning to optimize resources. As Michael J. Martens notes, "Protecting participant well-being is a core aspect in the implementation of clinical trials phase 2a and 2b studies," which emphasizes the ethical considerations that must guide stakeholder decisions.
Real-world examples, such as the BMT CTN 0601 study, demonstrate the vital role of risk evaluations in monitoring adverse events, reinforcing the necessity for comprehensive safety protocols. Furthermore, adaptive study designs can yield cost reductions of 10-15% by optimizing participant enrollment, making them an advantageous approach for stakeholders navigating the complexities of research trials.
As we look ahead to 2025, the importance of grasping these distinctions is more pertinent than ever. Stakeholders are eager to refine research strategies that lead to improved patient outcomes and increased drug approval rates. By leveraging insights from each phase, stakeholders can make informed decisions that significantly enhance the likelihood of successful clinical outcomes.
Understanding the distinctions between Phase 2a and Phase 2b clinical trials is crucial for shaping the future of medical research and treatment development. These differences empower stakeholders to make informed decisions that enhance the efficiency and effectiveness of clinical studies. Insights gained from these phases not only inform regulatory submissions but also pave the way for the advancement of promising therapies, ultimately benefiting patient care.
Key points highlighted throughout the article include:
Phase 2a trials focus on evaluating efficacy and safety in smaller participant groups, while Phase 2b trials expand the cohort size to optimize treatment regimens and assess broader effectiveness. The role of organizations like bioaccess® in facilitating these trials through cost-effective solutions and accelerated processes is significant, showcasing their contribution to overcoming common challenges in clinical research.
As the landscape of clinical trials continues to evolve, it is essential for stakeholders to leverage insights derived from both Phase 2a and Phase 2b studies. By doing so, they can refine research strategies, enhance participant safety, and ultimately drive forward the development of innovative treatments. Engaging with organizations like bioaccess® can be a pivotal step in navigating the complexities of clinical trials, ensuring that the journey from research to real-world impact is both efficient and successful.
What is bioaccess® and how does it impact clinical trials?
bioaccess® is a platform that accelerates Phase 2a and 2b clinical trials by leveraging Colombia's strategic advantages, offering cost savings of over 30% compared to North America and Western Europe, and enabling faster ethical approvals in just 4-6 weeks.
What are the benefits of conducting clinical trials in Colombia through bioaccess®?
Conducting trials in Colombia through bioaccess® allows for quicker recruitment from a population of over 50 million, with 95% covered by universal healthcare. It also achieves enrollment speeds that are 50% faster than in other regions and ensures high-quality data collection supported by Colombia's top-ranked healthcare system.
What is the objective of Phase 2a trials?
The objective of Phase 2a trials is to evaluate the efficacy and safety of treatments within a limited participant group, typically ranging from 20 to 100 individuals, and to identify adverse effects and initial efficacy.
How do Phase 2a trials contribute to drug development?
Phase 2a trials are critical for determining whether a treatment should progress to Phase 2b, providing insights that shape the design of subsequent phases and offering essential information for regulatory submissions.
What services does bioaccess® provide for Phase 2a studies?
bioaccess® offers comprehensive research study management services including feasibility assessments, site selection, compliance evaluations, study setup, and project oversight, along with meticulous reporting processes to monitor study status and adverse events.
What is the likelihood of success for Phase 2 assessments?
The overall likelihood of success for Phase 2 assessments is recognized to be at least 80%, highlighting their significance in the drug development landscape.
What is the focus of Phase 2b trials?
The focus of Phase 2b trials is to evaluate the effectiveness of treatments by exploring various dosages and optimizing treatment regimens while maintaining a strong emphasis on safety.
How does bioaccess® enhance the efficiency of Phase 2b trials?
bioaccess® enhances efficiency by achieving significant cost savings, allowing for earlier submission of PMA data, and integrating adaptive designs that enable real-time adjustments based on interim data.
Why is collaboration among stakeholders important in clinical research?
Collaboration among stakeholders is essential for navigating the complexities of clinical research and ensuring successful outcomes, particularly in overcoming recruitment challenges and designing effective studies.