


The article titled "10 Key Elements of a Clinical Trial Protocol Sample" serves to identify the essential components necessary for crafting an effective clinical trial protocol. A well-structured protocol is not just a formality; it includes critical elements such as study design, ethics, rationale, and safety considerations. These components are crucial for ensuring the trial's integrity and protecting participants, ultimately validating research outcomes. This structured approach facilitates successful medical advancements, underscoring the importance of meticulous planning in clinical research.
In the ever-evolving Medtech landscape, understanding these key elements becomes even more vital. As clinical trials face increasing scrutiny and complexity, the role of a robust protocol cannot be overstated. It addresses significant challenges, ensuring that trials are not only compliant but also effective in yielding reliable data. This is where organizations like Bioaccess step in, providing the expertise needed to navigate these complexities.
In conclusion, collaboration among stakeholders is essential for the success of clinical trials. By prioritizing the development of comprehensive protocols, researchers can enhance the quality of their studies and contribute to meaningful medical advancements. The next steps involve engaging with experts in the field to refine these protocols and ensure that they meet the highest standards of integrity and efficacy.
The complexities of clinical trial protocols can significantly influence the success or failure of innovative medical research. In a landscape where Medtech and biopharma are evolving rapidly, grasping the essential elements of a clinical trial protocol is more vital than ever. This article explores ten key components that not only streamline the trial process but also bolster participant safety and data integrity.
How can researchers ensure their protocols are not merely compliant but also optimized for efficiency and effectiveness in delivering life-saving treatments?
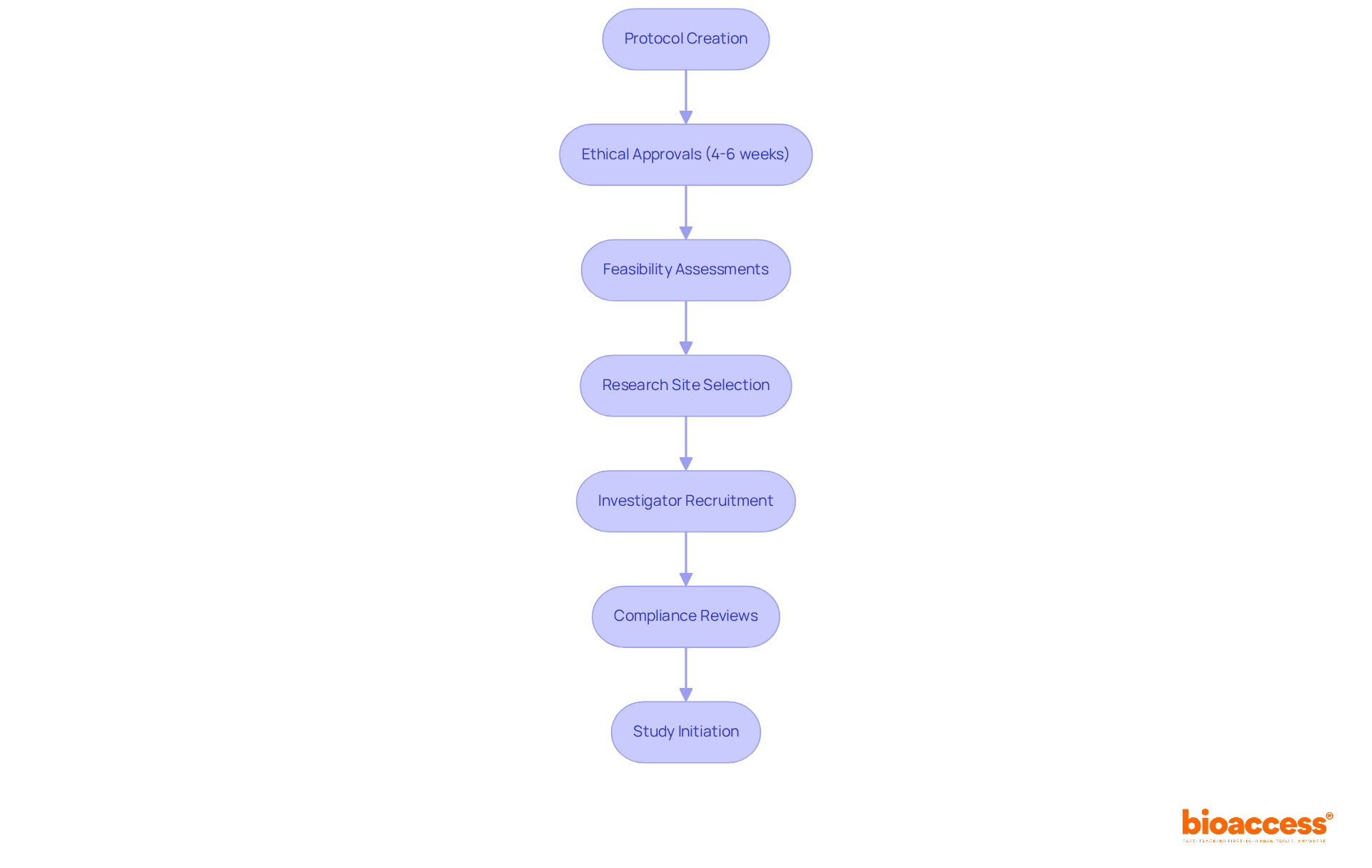
bioaccess® stands out in accelerating protocol development for studies by leveraging its deep understanding of regulatory frameworks across Latin America, the Balkans, and Australia. This expertise allows the organization to secure ethical approvals in an impressive 4-6 weeks, significantly shortening the timeline from protocol creation to study initiation. By streamlining processes like feasibility assessments, research site selection, investigator recruitment, and compliance reviews, bioaccess® not only speeds up medical studies but also empowers innovators in Medtech, Biopharma, and Radiopharma to hasten their market entry.
Industry leaders underscore the importance of regulatory knowledge for success. As one noted, "Sponsors can take steps to improve the site experience and thereby position themselves as a 'sponsor of choice.'" This sentiment is echoed by groups that have successfully reduced research initiation times through strategic partnerships and efficient procedures. The impact of robust regulatory frameworks on the speed of research initiation is profound, as they are essential for ensuring timely access to innovative treatments for patients in need.
The project summary is a crucial component of any research protocol, serving to clearly outline the main objectives and specific medical questions the study intends to address. This section should succinctly detail the target population, the intervention being tested, and the anticipated impact on patient care. A well-crafted project summary not only clarifies the project's purpose but also acts as a guiding reference throughout the research, ensuring that all activities remain aligned with the original goals.
Recent research underscores the importance of effective project summaries in enhancing study outcomes. They provide a clear structure for assessing success. For instance, directors of medical research emphasize that incorporating clear goals and expected outcomes in the project summary can lead to better alignment among stakeholders and improved resource distribution. Effective project summaries often highlight the significance of articulating study objectives in quantifiable terms, which fosters clearer communication and boosts the overall efficiency of the study.

The study design section delineates the methodology for clinical experiments and provides a clinical trial protocol sample, specifying whether the approach will be randomized, controlled, or observational. Detailing the sample size, recruitment methods, and the timeline for each phase of the clinical trial protocol sample is essential. For example, Phase I trials typically involve 20 to 80 individuals to assess safety, while Phase III trials may require hundreds to thousands to evaluate efficacy and monitor side effects. A well-structured research design not only bolsters the credibility of the findings but also facilitates smoother execution by providing clear guidelines for the research team.
Expert opinions highlight the importance of an adequate sample size and effective recruitment strategies in the context of a clinical trial protocol sample. A study lacking sufficient power may yield inconclusive results, whereas an overpowered study can squander resources and expose unnecessary participants to potential risks. Research specialists emphasize that achieving a balance in sample size, which can be illustrated in a clinical trial protocol sample, is crucial for reliable results, particularly for Medtech and biopharma startups facing unique recruitment challenges.
Successful research designs, such as the NRG-HN006 study, underscore the value of meticulous planning. This study randomizes patients with early-stage oral cancer to compare the effectiveness of sentinel lymph node biopsy against elective neck dissection, focusing on minimizing false-negative rates. Such examples illustrate how thoughtful design can lead to significant advancements in medical research, ultimately enhancing patient care.
Moreover, leveraging bioaccess®'s expertise in executing research studies in Latin America can significantly improve patient recruitment strategies. With a proven track record in managing Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies, bioaccess® offers a tailored approach that addresses the distinct challenges faced by Medtech and biopharma startups. Their ability to deliver FDA-ready data without rework or delays, as demonstrated in their Colombia study, enables faster patient recruitment at a fraction of the cost typically seen in the US. This not only streamlines the research process but also ensures that studies are conducted efficiently and effectively.

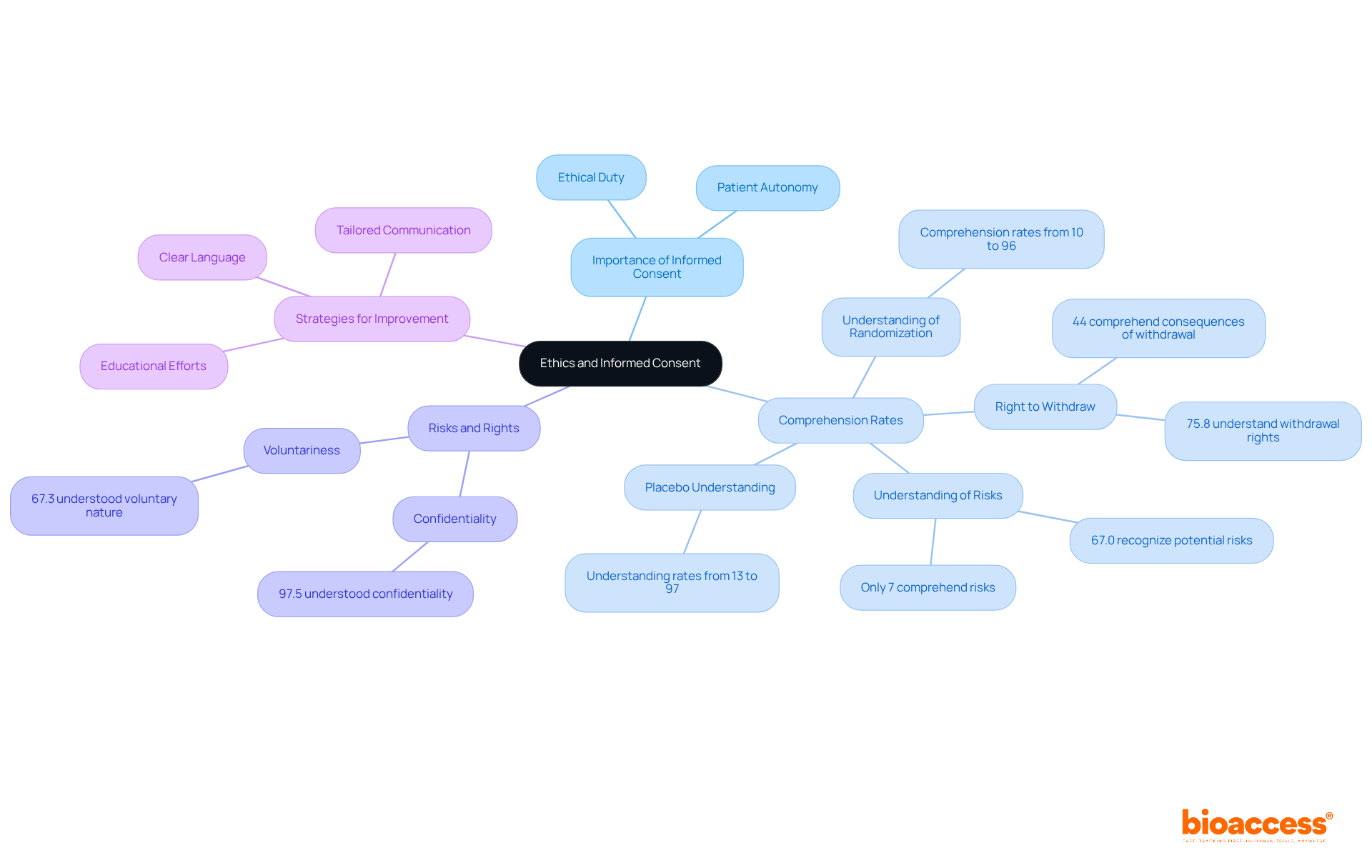
Ethical factors in medical studies are paramount, particularly regarding the informed consent process. Participants must be thoroughly informed about the study's purpose, procedures, risks, and benefits. This clarity is crucial; research indicates that only 7% of patients fully grasp the risks associated with clinical trials. A 2015 meta-analysis further revealed that 67.0% recognized the potential risks and side effects. This highlights a significant gap in understanding that must be addressed. To facilitate informed consent, researchers should employ clear, accessible language and provide comprehensive information that demystifies complex concepts such as randomization and placebo effects. For instance, understanding of randomization varies widely, with research showing comprehension rates ranging from 10% to 96%.
Moreover, measures to safeguard individuals are critical. These include ensuring confidentiality and clearly conveying individuals' rights, such as the right to withdraw from the study at any time without penalty. A systematic review found that while 75.8% of individuals understood their right to withdraw, awareness of the consequences of withdrawal was significantly lower, with only 44% demonstrating comprehension. This discrepancy underscores the need for enhanced educational efforts during the consent process.
Informed consent is not merely a formality; it is a fundamental ethical duty that honors individual autonomy. Bioethicists emphasize that the integrity of medical research relies on the ability of participants to make informed choices. As one specialist remarked, "The ethical basis of research trials is established on the assumption that individuals fully comprehend the informed consent they offer." Nguyen Tien Huy further highlights that understanding varies significantly among individuals, indicating that tailored strategies are necessary to enhance comprehension. Therefore, it is crucial for investigators to adopt strategies that improve participants' understanding, thereby fostering trust and ensuring ethical compliance in clinical research.
The rationale and background section must provide a comprehensive overview of the existing literature relevant to the study's focus. It should express the gaps in existing knowledge that the study intends to address, thereby emphasizing the importance of the research question. Establishing a strong rationale not only justifies the necessity of the experiment but also places it within the broader context of medical advancements.
For instance, Avantec Vascular's first-in-human trial of an innovative vascular device in Latin America, backed by bioaccess™, illustrates the continuous progress in vascular medicine. This research seeks to address significant gaps in understanding the effectiveness and safety of new vascular therapies while emphasizing the importance of thorough management services for comprehensive evaluations. These evaluations include:
By incorporating insights from earlier studies, researchers can recognize discrepancies and gaps, underscoring the significance of a well-organized literature review in justifying research questions related to medical studies. Moreover, the regulatory environment influenced by organizations such as INVIMA is vital in guaranteeing that studies are not only scientifically valid but also ethically sound.
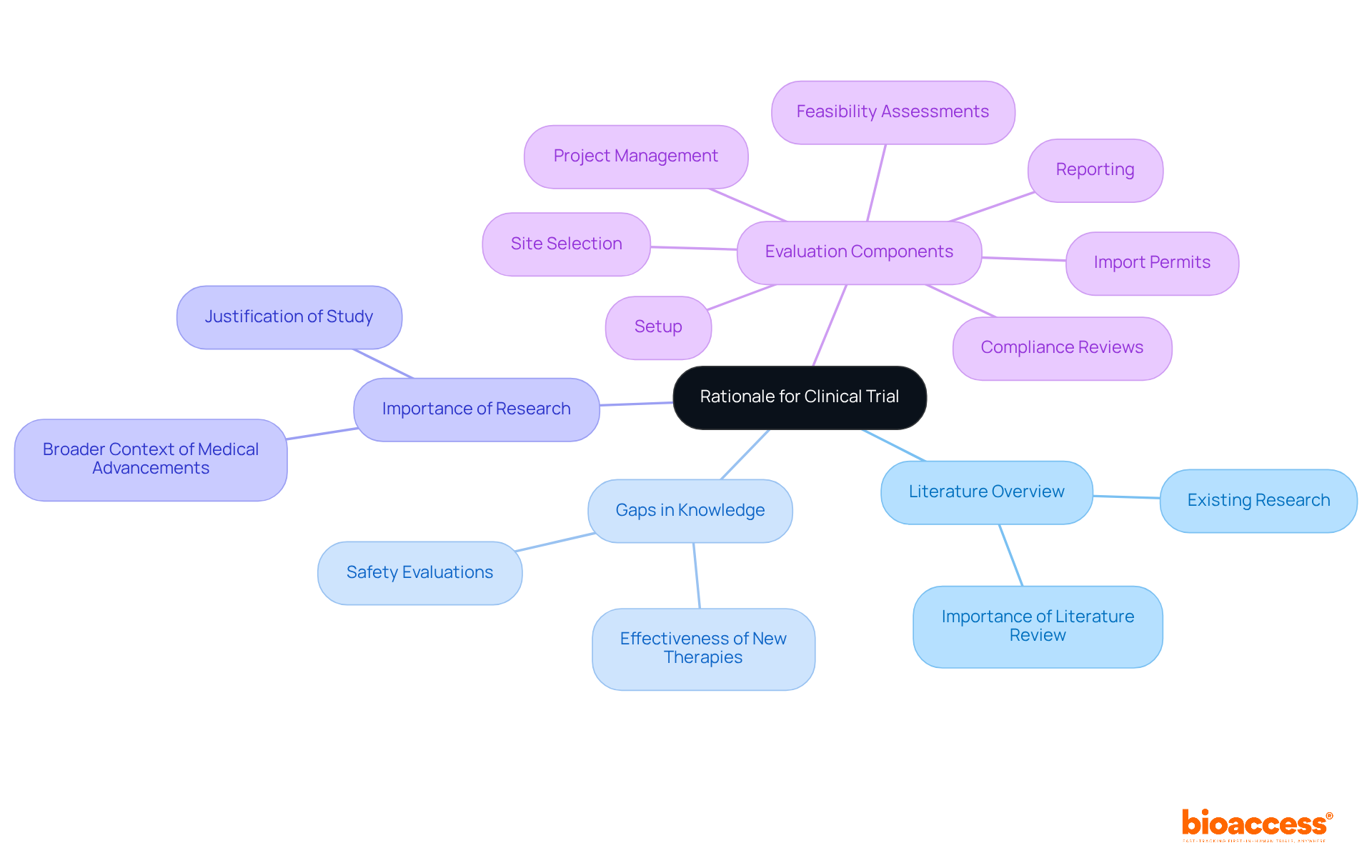
Safety factors must be a priority in any clinical trial protocol sample. This section outlines the essential procedures for monitoring the safety of individuals involved in the clinical trial protocol sample, including the establishment of Data Safety Monitoring Boards (DSMBs) and the criteria for reporting adverse events. At bioaccess, we emphasize extensive management services for a clinical trial protocol sample that encompass feasibility assessments and site selection, ensuring a favorable research environment for participant safety.
Our compliance evaluations and experimental setup procedures, outlined in the clinical trial protocol sample, are designed to conform to regulatory standards, significantly reducing risks. Additionally, we implement a clinical trial protocol sample as part of our thorough project management and reporting protocols to effectively monitor study status and adverse events. By prioritizing safety and incorporating these robust management practices outlined in the clinical trial protocol sample, researchers can safeguard participants and maintain the integrity of the study.

A well-organized data management strategy is essential for clinical studies, clearly outlining how data will be gathered, stored, and analyzed. This plan must detail the statistical methods employed to assess study outcomes, ensuring alignment with the study design. Common statistical analysis methods, such as regression analysis, survival analysis, and mixed models, play a crucial role in interpreting complex data sets and drawing valid conclusions.
Looking ahead to 2025, trends in statistical analysis methods are increasingly favoring adaptive designs and Bayesian approaches. These innovative methods provide researchers with the flexibility to make real-time adjustments based on interim results, significantly enhancing the study's responsiveness to emerging data.
Biostatisticians stress the importance of selecting appropriate statistical techniques to ensure valid results. As one expert noted, "The selection of statistical techniques can greatly influence the interpretation of research outcomes, making it essential to align them with the study's goals and framework." By implementing robust data management and analysis strategies, researchers can bolster the validity of their results, ultimately leading to more reliable conclusions and advancements in medical science.

The anticipated outcomes section must clearly outline the primary and secondary endpoints of the study. These metrics serve as vital benchmarks for assessing the intervention's effectiveness. By setting defined success standards, researchers can evaluate whether the study meets its goals, offering valuable insights into medical practices. Recent studies indicate that clearly defined endpoints can significantly enhance the reliability of study outcomes, focusing on both primary and secondary metrics.
For instance, primary endpoints often measure direct clinical effects, such as overall survival or disease progression. In contrast, secondary endpoints may assess additional benefits or side effects, providing a comprehensive view of the intervention's impact. Clinical researchers emphasize that clarity in endpoint definitions not only aids in regulatory compliance but also fosters better communication among stakeholders. This ultimately leads to improved study designs and outcomes.
By prioritizing the establishment of these success metrics, researchers can ensure that their studies contribute meaningfully to the advancement of medical knowledge and patient care. The importance of collaboration in this process cannot be overstated, as it paves the way for innovative solutions to the challenges faced in clinical research.

Distributing research findings is crucial for ensuring that acquired knowledge reaches those who can benefit from it, thereby advancing medical practice and improving patient care. Effective strategies for sharing findings include:
Alarmingly, statistics reveal that only 29% of finalized research studies from leading academic institutions were published within two years, underscoring the urgent need for better reporting practices. Furthermore, the overall distribution rate of research findings stands at an estimated 54%, with significant variation across institutions.
Researchers bear a moral obligation to share their discoveries; failing to do so can jeopardize evidence-based medical decision-making and violate commitments to study participants. As one expert noted, "Prompt sharing of the results of medical studies is crucial to assist evidence-based choices by patients and providers." Additionally, media coverage plays a vital role in informing the public and stakeholders about research studies, particularly in regions like Latin America and Colombia, where such information can influence local economies and healthcare advancements.
Sharing results not only fulfills ethical responsibilities but also enhances transparency and fosters trust among stakeholders. This, in turn, contributes to the advancement of medical knowledge and improves patient outcomes. What steps can we take to ensure that research findings are effectively communicated and utilized?

Efficient budget and resource distribution are crucial for the success of clinical studies, requiring a comprehensive analysis of the financial resources necessary for the research. This encompasses personnel costs, equipment expenses, and operational expenditures. A well-organized budget not only ensures that all aspects of the study are adequately funded but also positions the research for optimal outcomes.
Strategic resource allocation involves pinpointing key areas where funding is most critical and prioritizing these in the budget. For instance, personnel expenses typically account for a significant portion of the budget, often including salaries, benefits, and training for the staff involved in the study. Moreover, operational costs, which cover site management and patient care, can represent up to 30% of the total budget.
To secure funding, researchers should explore various sources, such as government grants, private investments, and partnerships with pharmaceutical companies. A recent analysis indicated that nearly 40% of the US pharmaceutical research budget—approximately $7 billion annually—is allocated for research studies, underscoring the substantial financial commitment required.
Clinical research directors emphasize the necessity of securing adequate funding: "Effective budget management not only guarantees that studies are financially feasible but also enhances return on investment (ROI)." This statement highlights the importance of aligning budget strategies with the overarching objectives of the experiment.
Examples of effective resource allocation strategies include:
In summary, meticulous planning and strategic resource allocation are vital for navigating the complexities of a clinical trial protocol sample, ensuring the trial is well-supported and positioned for success.

The significance of a well-structured clinical trial protocol cannot be overstated; it serves as the backbone of successful research endeavors. Each key element—from the project summary to budget considerations—plays an integral role in ensuring that trials are conducted efficiently, ethically, and with the ultimate goal of advancing medical knowledge and patient care.
Throughout this article, essential components such as:
have been highlighted. These elements collectively contribute to the robustness of clinical research, ensuring that studies are not only scientifically valid but also ethically sound and aligned with the needs of stakeholders. The insights provided emphasize the importance of meticulous planning and effective communication in achieving successful outcomes.
Given the critical role that clinical trial protocols play, stakeholders in the medical research community must prioritize these elements in their planning and execution processes. By doing so, they can enhance the quality of their studies, accelerate the delivery of innovative treatments, and ultimately improve patient outcomes. Embracing the best practices outlined in this article will pave the way for more efficient and impactful clinical research, fostering a healthier future for all.
What is bioaccess® and how does it accelerate clinical trial protocol development?
bioaccess® is an organization that accelerates protocol development for clinical studies by leveraging its deep understanding of regulatory frameworks in regions such as Latin America, the Balkans, and Australia. This expertise enables them to secure ethical approvals in 4-6 weeks, significantly shortening the timeline from protocol creation to study initiation.
What processes does bioaccess® streamline to speed up medical studies?
bioaccess® streamlines several processes including feasibility assessments, research site selection, investigator recruitment, and compliance reviews. This not only speeds up medical studies but also helps innovators in Medtech, Biopharma, and Radiopharma to hasten their market entry.
Why is regulatory knowledge important for clinical trials?
Regulatory knowledge is crucial for clinical trials as it helps sponsors improve the site experience and position themselves as a 'sponsor of choice.' Robust regulatory frameworks significantly impact the speed of research initiation, ensuring timely access to innovative treatments for patients.
What is the purpose of the project summary in a clinical trial?
The project summary is a vital component of a research protocol that outlines the main objectives and specific medical questions the study intends to address. It details the target population, the intervention being tested, and the anticipated impact on patient care, serving as a guiding reference throughout the research.
How do effective project summaries enhance study outcomes?
Effective project summaries enhance study outcomes by providing a clear structure for assessing success. They help align stakeholders and improve resource distribution by incorporating clear goals and expected outcomes.
What is included in the study design section of a clinical trial protocol?
The study design section outlines the methodology for clinical experiments, specifying whether the approach will be randomized, controlled, or observational. It details the sample size, recruitment methods, and the timeline for each phase of the clinical trial.
Why is sample size important in clinical trials?
Sample size is important because an inadequate sample size may yield inconclusive results, while an overpowered study can waste resources and expose unnecessary participants to risks. Achieving a balance in sample size is crucial for obtaining reliable results.
How does bioaccess® improve patient recruitment strategies?
bioaccess® improves patient recruitment strategies by leveraging its expertise in executing research studies in Latin America. Their tailored approach addresses the unique challenges faced by Medtech and biopharma startups, enabling faster patient recruitment at a lower cost compared to traditional methods in the US.