


Navigating the labyrinth of clinical trial regulations can be daunting, particularly in a rapidly evolving landscape like Bulgaria’s. The introduction of Early Access Programs (EAP) makes it essential for researchers and sponsors to grasp the intricacies of compliance. This article explores ten key insights that shed light on the current trends, challenges, and best practices surrounding EAP regulations in Bulgaria, providing a roadmap for successful clinical trials.
What strategies can stakeholders implement to not only meet regulatory demands but also enhance patient access to innovative therapies?
Bioaccess® leverages its extensive research experience to simplify compliance with EAP regulations for Bulgaria. This strategic approach not only accelerates the approval process-targeting review durations of just 35 days by the Bulgarian Drug Agency-but also significantly enhances the quality of studies conducted in the region. With over 50 sites activated in under 8 weeks and a robust patient recruitment network, Bulgaria stands out as a prime destination for sponsors aiming for rapid and compliant study execution.
In the evolving Medtech landscape, bioaccess® plays a crucial role in addressing the complexities of the EAP regulations for Bulgaria. By utilizing in-depth local knowledge and regulatory expertise, the company empowers Medtech, Biopharma, and Radiopharma innovators to navigate these challenges effectively. As Tatyana Benisheva aptly points out, "These medical studies serve as extra therapy alternatives and health resources for the healthcare systems in the EU-EECs, as well as a means to offer patients access to innovative and novel therapies that cannot be financed by public budget funds."
The collaboration between bioaccess® and its partners is vital for advancing clinical research in Bulgaria. By fostering an environment of innovation and compliance, bioaccess® not only supports the healthcare system but also enhances patient access to groundbreaking therapies. As the Medtech sector continues to grow, the importance of such partnerships cannot be overstated.
What challenges do you face in navigating the EAP regulations for Bulgaria? Let bioaccess® guide you through the complexities and help you achieve your research goals.

In Bulgaria, compliance with the EU Clinical Studies Regulation (EU No 536/2014) is essential for conducting clinical research. This regulation mandates that all experiments obtain ethical approval from a recognized ethics committee, which is vital for protecting participant rights and ensuring ethical research conduct. Informed consent from participants is a fundamental requirement, alongside strict adherence to data protection laws to uphold confidentiality and integrity.
Sponsors are required to submit detailed study protocols and safety reports to the Bulgarian Drug Agency (BDA) for comprehensive review. This process enhances the reliability of research studies while promoting transparency and accountability within the research framework. Notably, approximately 550 research studies are currently active in Bulgaria, with a significant percentage successfully obtaining ethical approval, showcasing the nation's commitment to high standards in medical research.
The efficiency of ethical approval procedures in Bulgarian studies is evident, as the centralized Clinical Trials Information System (CTIS) streamlines submissions and reduces review times. This regulatory environment, combined with Bulgaria's skilled workforce and robust healthcare infrastructure, positions the country as an attractive destination for medical research, ensuring that ethical considerations are prioritized throughout the study lifecycle.

In Bulgaria, the ethical approval procedure stands as a pivotal phase in conducting research studies, requiring a thorough application to be submitted to an ethics committee. This committee rigorously evaluates the study's design, objectives, and potential risks to participants, ensuring that ethical standards are maintained. A primary focus is on the adequacy of informed consent procedures, which are vital for protecting participant welfare.
Notably, the Bulgarian Drug Agency aims for a review timeline of just 35 days for trial applications, positioning the country among the fastest in the EU for ethical approvals. By 2025, this efficiency is anticipated to significantly enhance the clinical research landscape in Bulgaria. Here, bioaccess® plays an essential role by aiding sponsors in preparing their applications, thus facilitating timely approvals and ensuring adherence to ethical standards.
This support is particularly beneficial, given that 95% of Cromos Pharma's studies meet or surpass enrollment goals within expected timelines. This statistic underscores the effectiveness of local expertise in navigating the ethical approval landscape. As the clinical research environment evolves, collaboration and strategic partnerships will be crucial for continued success.

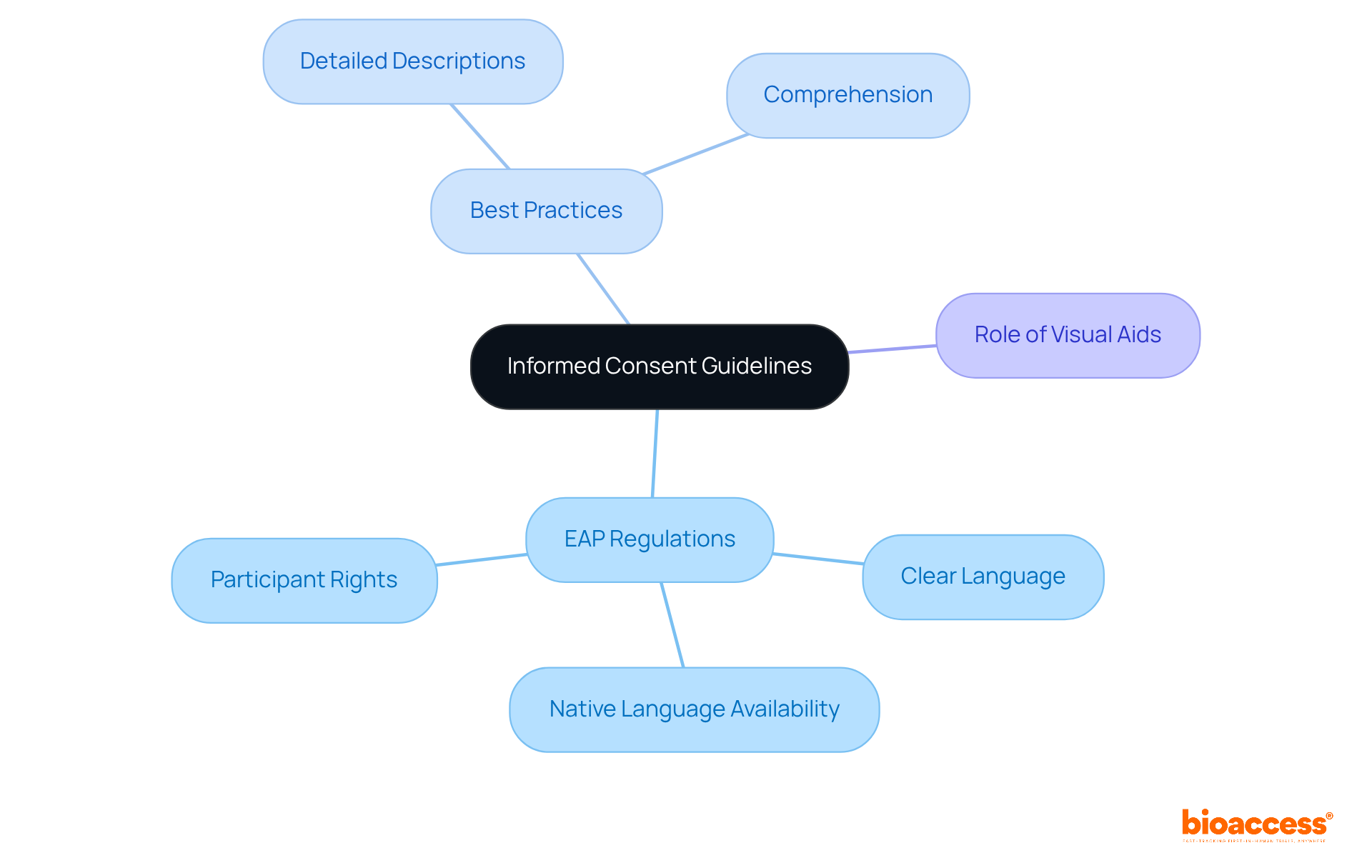
Informed consent stands as a cornerstone of clinical research, ensuring that participants are thoroughly informed about the study's purpose, procedures, risks, and benefits before agreeing to participate. Under the EAP regulations for Bulgaria, it is required that consent forms be clear, concise, and available in the participant's native language. Compliance experts emphasize that effective consent materials should not only meet these standards but also enhance participant understanding.
To create optimal informed consent documents, it is essential to use clear language, provide detailed descriptions of study procedures, and ensure that participants fully comprehend their rights, including the right to withdraw at any time. Effective consent materials often incorporate visual aids and interactive formats, which significantly facilitate understanding. At Bioaccess®, we prioritize transparency and clarity in the informed consent process, assisting sponsors in developing materials that not only meet regulatory requirements but also foster a deeper understanding among participants.
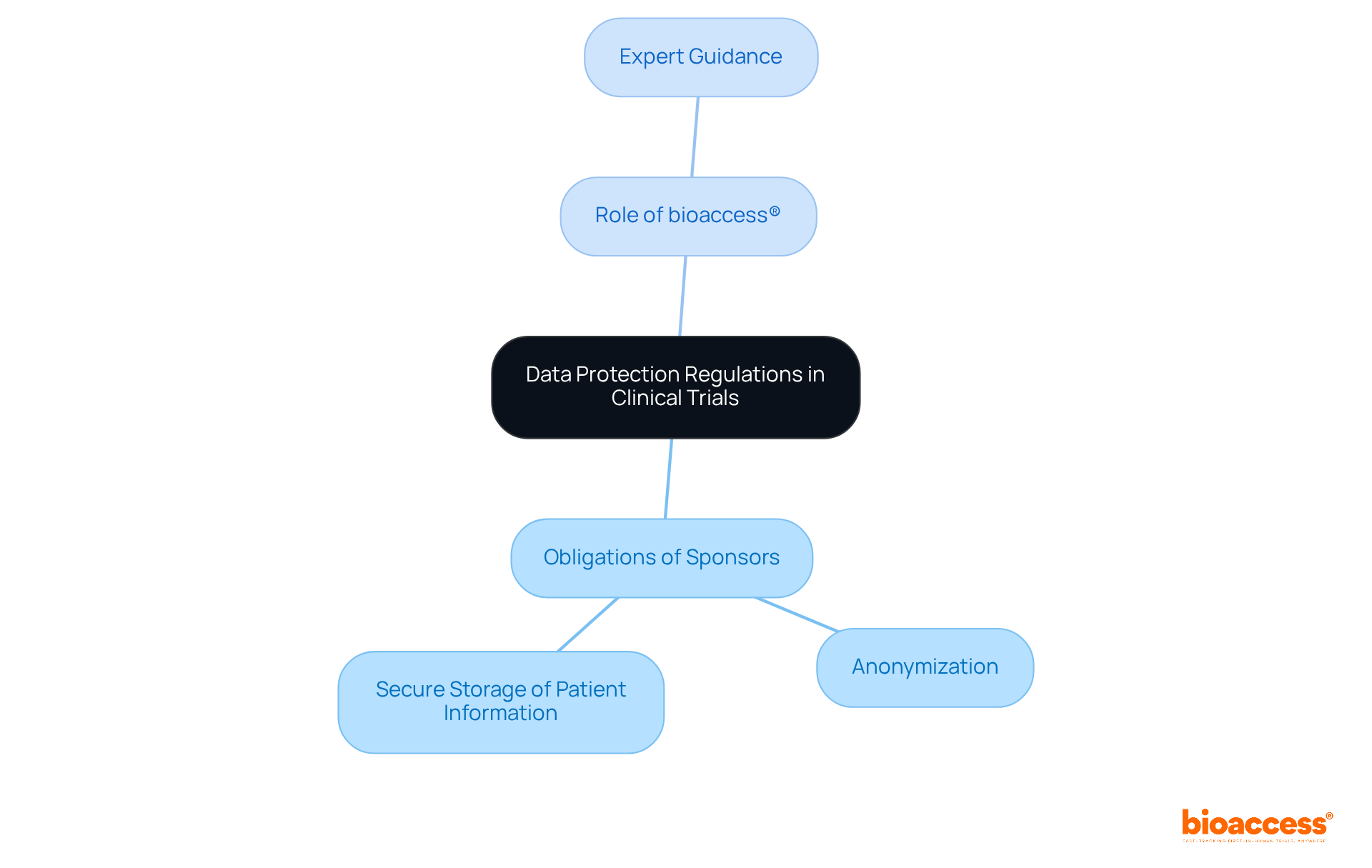
Data protection regulations in Bulgaria are primarily governed by the General Data Protection Regulation (GDPR) alongside local laws. These regulations are crucial for clinical research, as they mandate that personal information collected during studies must be handled legally, transparently, and securely. Sponsors are obligated to implement robust data protection measures, which include:
bioaccess® plays a pivotal role in this landscape by providing expert advice on adhering to these regulations. Our guidance ensures that sponsors can effectively safeguard participant data while conducting their studies, addressing key challenges in the Medtech sector. By prioritizing data protection, sponsors not only comply with legal requirements but also build trust with participants, enhancing the overall integrity of their research.
In conclusion, collaboration with experts like bioaccess® is essential for navigating the complexities of data protection in clinical research. We encourage sponsors to take proactive steps in implementing these measures, ensuring that participant data is treated with the utmost care and respect.
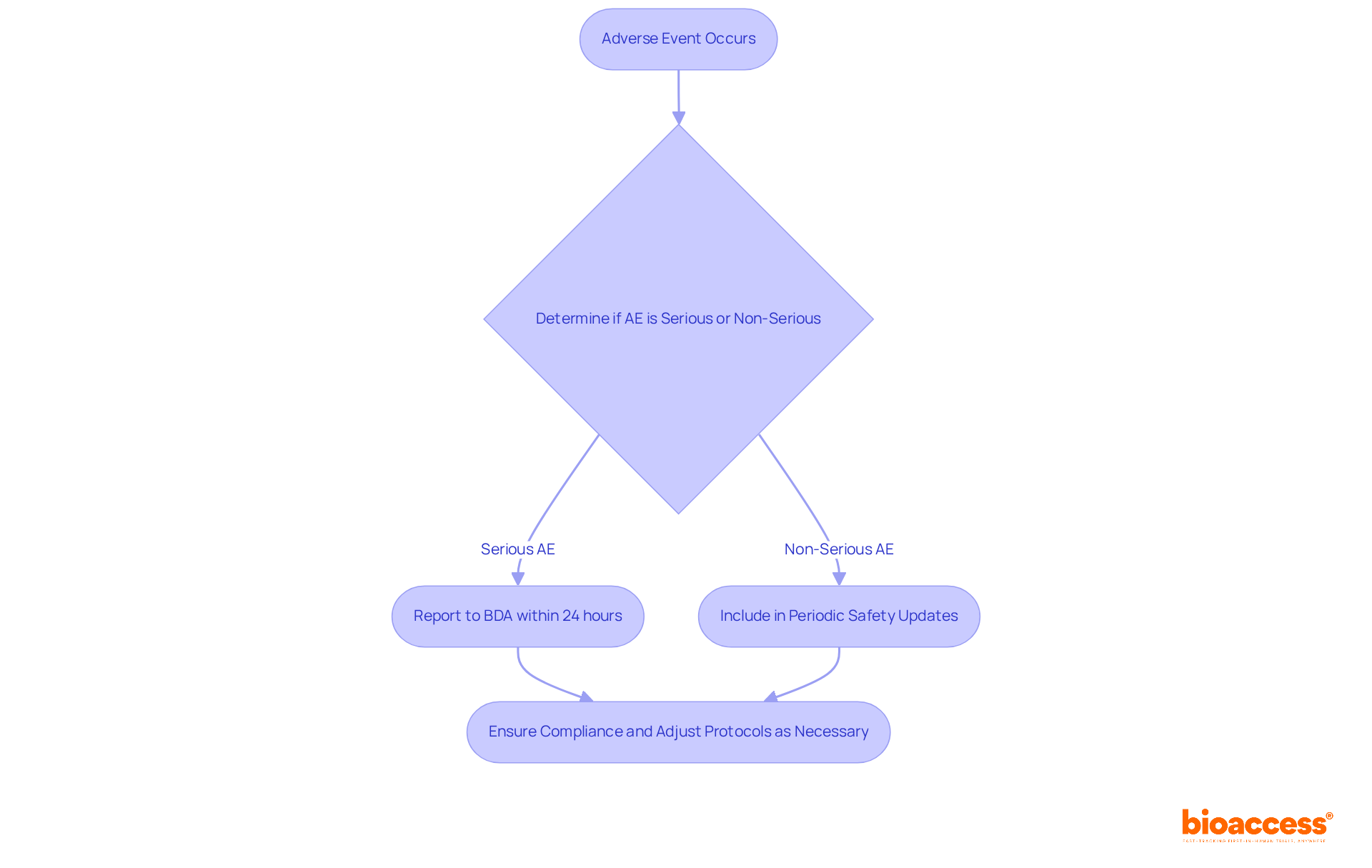
In Bulgaria, sponsors must report all adverse events (AEs) that occur during research studies to the Bulgarian Drug Agency (BDA) and relevant ethics committees. This requirement includes both serious adverse events (SAEs) and non-serious adverse events (NSAEs), with strict timelines for reporting. For example, SAEs typically need to be reported within 24 hours, while non-serious AEs can be included in periodic safety updates. Adhering to these responsibilities is crucial for safeguarding participant safety and upholding the integrity of research studies.
Timely reporting not only helps in identifying potential risks but also ensures that necessary adjustments to study protocols can be implemented swiftly. As highlighted by industry experts, failing to report AEs promptly can lead to significant penalties, testing delays, or even suspension of the research study. This is where bioaccess® plays a vital role, assisting sponsors in establishing robust adverse event reporting systems. By ensuring that submissions are both timely and accurate, bioaccess® enhances overall study safety and compliance with the eap regulations for Bulgaria.
In the ever-evolving Medtech landscape, collaboration is key. By working together, sponsors and service providers can navigate the complexities of clinical research more effectively. The next steps involve leveraging these insights to strengthen reporting systems and ensure participant safety remains a top priority.
The regulatory environment for clinical studies in Bulgaria is dynamic, making it essential for sponsors to stay alert to updates regarding the EAP regulations for Bulgaria. Key authorities, such as the Bulgarian Drug Agency and the European Medicines Agency, frequently issue updates that can significantly impact research operations. Notably, as of January 31, 2025, all ongoing studies must transition to the Clinical Trials Information System (CTIS). This shift aims to streamline processes and enhance transparency, which is crucial for maintaining the integrity of clinical research.
Moreover, the Bulgarian Drug Agency has set a goal for evaluations of studies to be completed within 35 days of successful application validation, showcasing a commitment to efficiency. With over 1,000 hospitals and research facilities available, sponsors have ample opportunities to conduct trials effectively. However, navigating these legislative changes can be challenging. This is where bioaccess® steps in, offering customized solutions that include approval processes, patient recruitment, and expert guidance on adhering to the latest regulations and best practices.
This proactive approach is vital for ensuring the success of research initiatives in Bulgaria. By collaborating with bioaccess®, sponsors can not only meet regulatory demands but also enhance their operational efficiency and research outcomes.

Local oversight bodies, especially the Bulgarian Drug Agency (BDA), are pivotal in enforcing adherence to early access programs (EAP) in clinical research. The BDA meticulously oversees the approval process, ensuring that all studies align with established regulations and ethical standards. In 2024, the BDA set an ambitious target of a 35-day approval timeline for clinical trial applications, significantly boosting Bulgaria's attractiveness as a trial location. Furthermore, the agency conducts routine inspections and audits to ensure compliance, which is essential for maintaining high data quality and regulatory adherence.
bioaccess® collaborates closely with the BDA to streamline interactions, enabling sponsors to navigate the regulatory landscape effectively and meet all necessary requirements. This partnership not only facilitates quicker approvals but also fortifies the integrity of research conducted in Bulgaria. With bioaccess's comprehensive services-including:
the company plays a crucial role in expediting research phases while ensuring adherence to FDA/EMA/MDR standards across LATAM, Eastern Europe, and Australia.

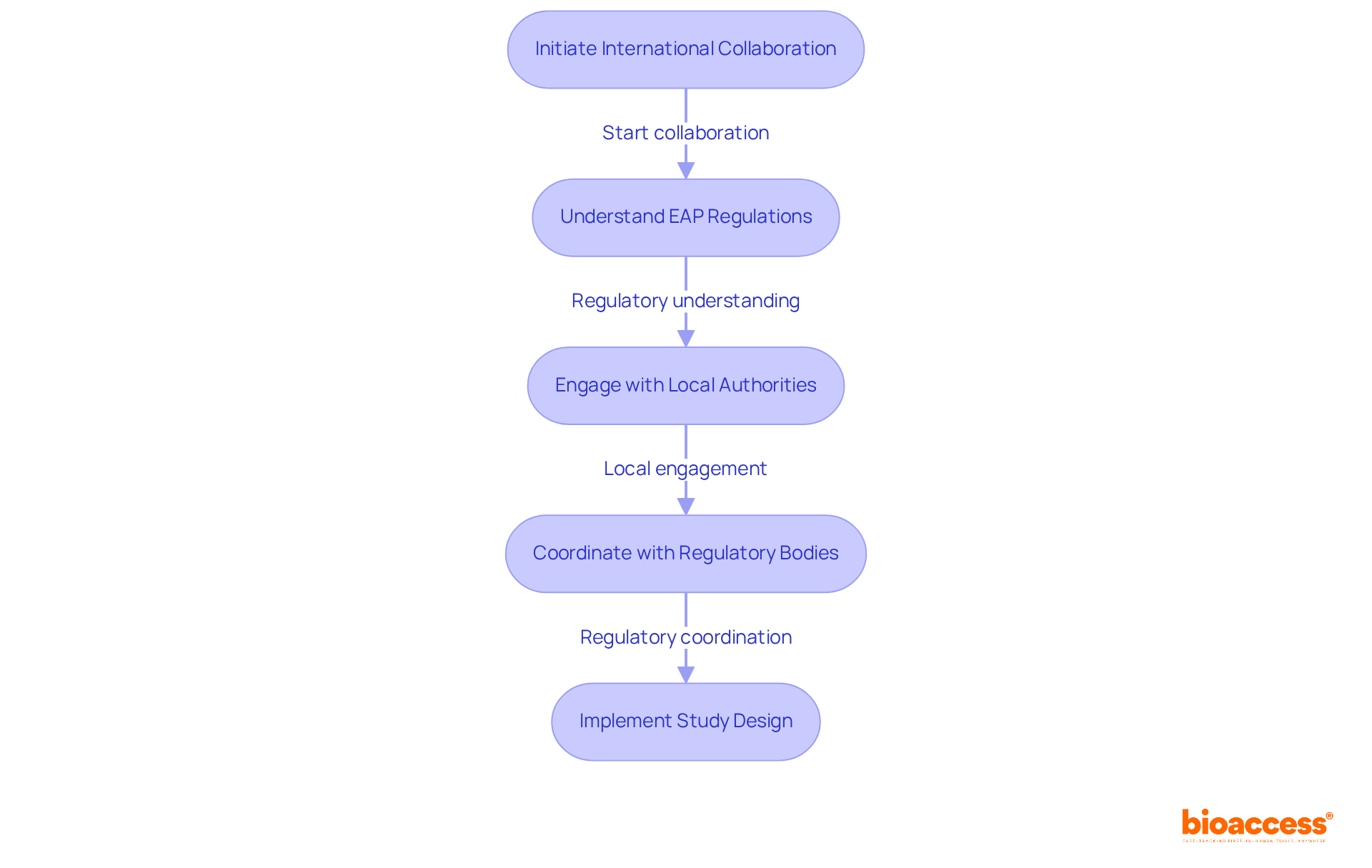
Conducting clinical studies across borders presents significant complexities, particularly concerning the EAP regulations for Bulgaria. Sponsors must adeptly navigate a landscape filled with varying legal requirements and ethical standards across different jurisdictions. Clear and timely communication is essential for overcoming these challenges, as emphasized by industry experts. For instance, Sandra Goldsworthy highlights the importance of establishing strong international research collaborations to enhance patient safety and improve healthcare practices.
bioaccess® plays a crucial role in overseeing these global partnerships, ensuring that sponsors adhere to local regulations while maintaining the integrity of their studies. This involves coordinating with regulatory bodies across multiple jurisdictions to streamline the approval process. Specific services offered by bioaccess include:
All critical in facilitating these collaborations. A notable example is the partnership between bioaccess® and Caribbean Health Group, which aims to position Barranquilla as a premier location for medical studies in Latin America. This partnership, supported by Colombia's Minister of Health, exemplifies how strategic alliances can promote adherence and enhance the clinical trial landscape.
The complexities of cross-border regulations are further compounded by the necessity of understanding each country's specific EAP regulations for Bulgaria. Chris Moore points out that navigating these regulatory challenges requires careful planning and proactive engagement with local authorities.
As we look ahead to 2025, the environment of international research studies continues to evolve, with an increasing focus on adherence and cooperation. The challenges of ensuring compliance with diverse regulations can be daunting, yet with the right strategies in place, sponsors can successfully navigate these complexities. Effective navigation of EAP regulations for Bulgaria, as demonstrated by initiatives led by bioaccess®, showcases the potential for successful international partnerships, ultimately resulting in more efficient research processes and improved patient outcomes.
Investing in training and resources is essential for clinical study teams to ensure adherence to the EAP regulations for Bulgaria. This investment encompasses education on regulatory requirements, ethical standards, and effective study methodologies. Customized training programs offered by bioaccess® empower teams to proficiently navigate the EAP regulations for Bulgaria.
Studies indicate that organizations with comprehensive training programs achieve significantly higher adherence rates, leading to improved trial quality and efficiency. Furthermore, bioaccess® provides complementary services such as feasibility studies and project management, enhancing these training initiatives. By cultivating a culture of compliance, sponsors not only boost their operational effectiveness but also uphold the integrity of clinical research in Bulgaria.

In summary, understanding and navigating the EAP regulations for clinical trials in Bulgaria is essential for sponsors who aim to conduct research efficiently and ethically. This article underscores the pivotal role of bioaccess® in ensuring compliance, streamlining processes, and enhancing study quality. By leveraging local expertise and regulatory knowledge, bioaccess® not only accelerates the approval timeline but also establishes a robust framework for ethical research practices.
Key insights discussed include the significance of:
The collaboration with local regulatory authorities, such as the Bulgarian Drug Agency, emerges as a vital component in ensuring adherence to compliance standards. As the clinical research landscape evolves, staying updated on regulatory changes and investing in training resources will be crucial for maintaining high standards in medical research.
The importance of these insights cannot be overstated. By fostering collaboration, prioritizing compliance, and utilizing expert guidance, sponsors can effectively navigate the complexities of EAP regulations. Embracing these practices not only enhances research outcomes but also contributes to advancing patient access to innovative therapies in Bulgaria and beyond.
What is bioaccess® and its role in Bulgaria?
Bioaccess® is a company that simplifies compliance with Expanded Access Program (EAP) regulations in Bulgaria, accelerating the approval process to target review durations of just 35 days by the Bulgarian Drug Agency.
How does bioaccess® enhance the quality of studies in Bulgaria?
Bioaccess® enhances the quality of studies by leveraging extensive research experience and a robust patient recruitment network, having activated over 50 sites in under 8 weeks.
What are the key requirements for compliance with clinical trials in Bulgaria?
Compliance with the EU Clinical Studies Regulation (EU No 536/2014) is essential, which includes obtaining ethical approval from a recognized ethics committee, ensuring informed consent from participants, and adhering to data protection laws.
What is the significance of ethical approval in Bulgarian research studies?
Ethical approval is crucial for protecting participant rights and ensuring ethical research conduct, as it involves a rigorous evaluation of the study's design, objectives, and potential risks.
How long does the ethical approval process take in Bulgaria?
The Bulgarian Drug Agency aims for a review timeline of just 35 days for trial applications, making it one of the fastest in the EU for ethical approvals.
What is the role of the Clinical Trials Information System (CTIS) in Bulgaria?
The CTIS streamlines submissions and reduces review times, enhancing the efficiency of ethical approval procedures for clinical studies in Bulgaria.
How does bioaccess® support sponsors in the ethical approval process?
Bioaccess® aids sponsors in preparing their applications for ethical approval, facilitating timely approvals and ensuring adherence to ethical standards.
What is the current status of research studies in Bulgaria?
Approximately 550 research studies are currently active in Bulgaria, with a significant percentage successfully obtaining ethical approval, highlighting the country's commitment to high standards in medical research.
Why is Bulgaria considered an attractive destination for medical research?
Bulgaria's skilled workforce, robust healthcare infrastructure, and efficient regulatory environment make it an attractive destination for conducting medical research.