


Navigating the complex landscape of clinical research in Bosnia and Herzegovina demands a strong framework of ethical oversight, particularly through the lens of ethics board standard operating procedures (SOPs). These SOPs are essential not only for ensuring compliance with local regulations and international ethical standards but also for protecting the rights and welfare of research participants. Yet, with Bosnia recording the lowest number of clinical trials in the Balkans, a pressing question arises: how can ethics boards enhance their practices to build greater trust and encourage participation in clinical research?
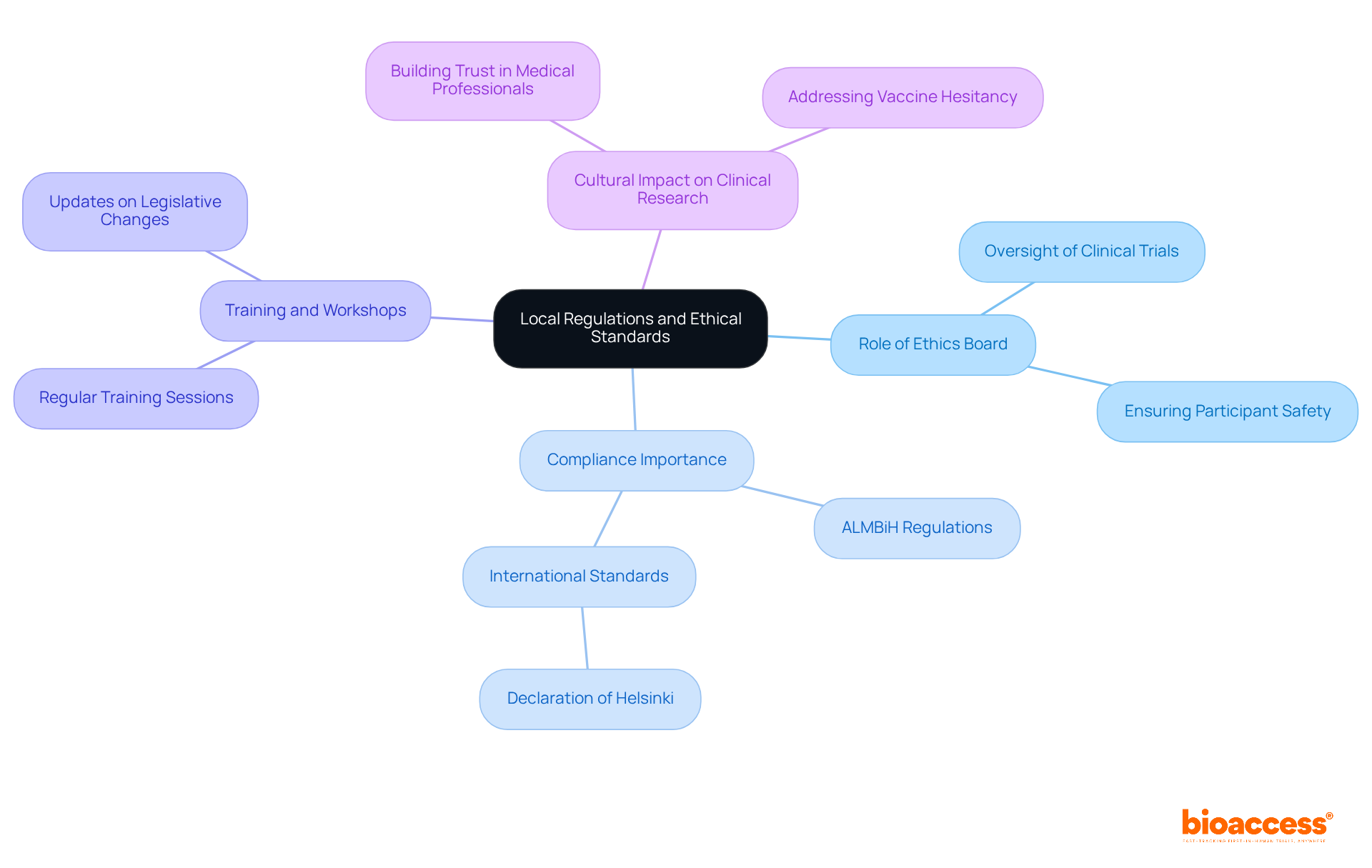
The ethics board sops in Bosnia and Herzegovina play a crucial role in navigating the regulatory landscape that governs clinical research. This landscape is shaped by the Agency for Medicinal Products and Medical Devices (ALMBiH) and international standards, such as the Declaration of Helsinki. Compliance with these regulations is not just a formality; it is essential for protecting the rights and welfare of research participants.
Statistics reveal that Bosnia has the lowest number of clinical trials in the Balkans, highlighting a pressing need for robust ethical oversight. This oversight is vital for enhancing participation and building trust in clinical research. To ensure that standard operating practices align with these regulations, regular training sessions and workshops must be prioritized by the ethics board sops in Bosnia and Herzegovina.
This proactive approach keeps directors informed about legislative changes and fosters a culture of compliance and ethical integrity. Such a culture is indispensable for advancing clinical research in the region. By investing in education and training, ethics committees can significantly improve the landscape of clinical trials, ultimately benefiting both researchers and participants.
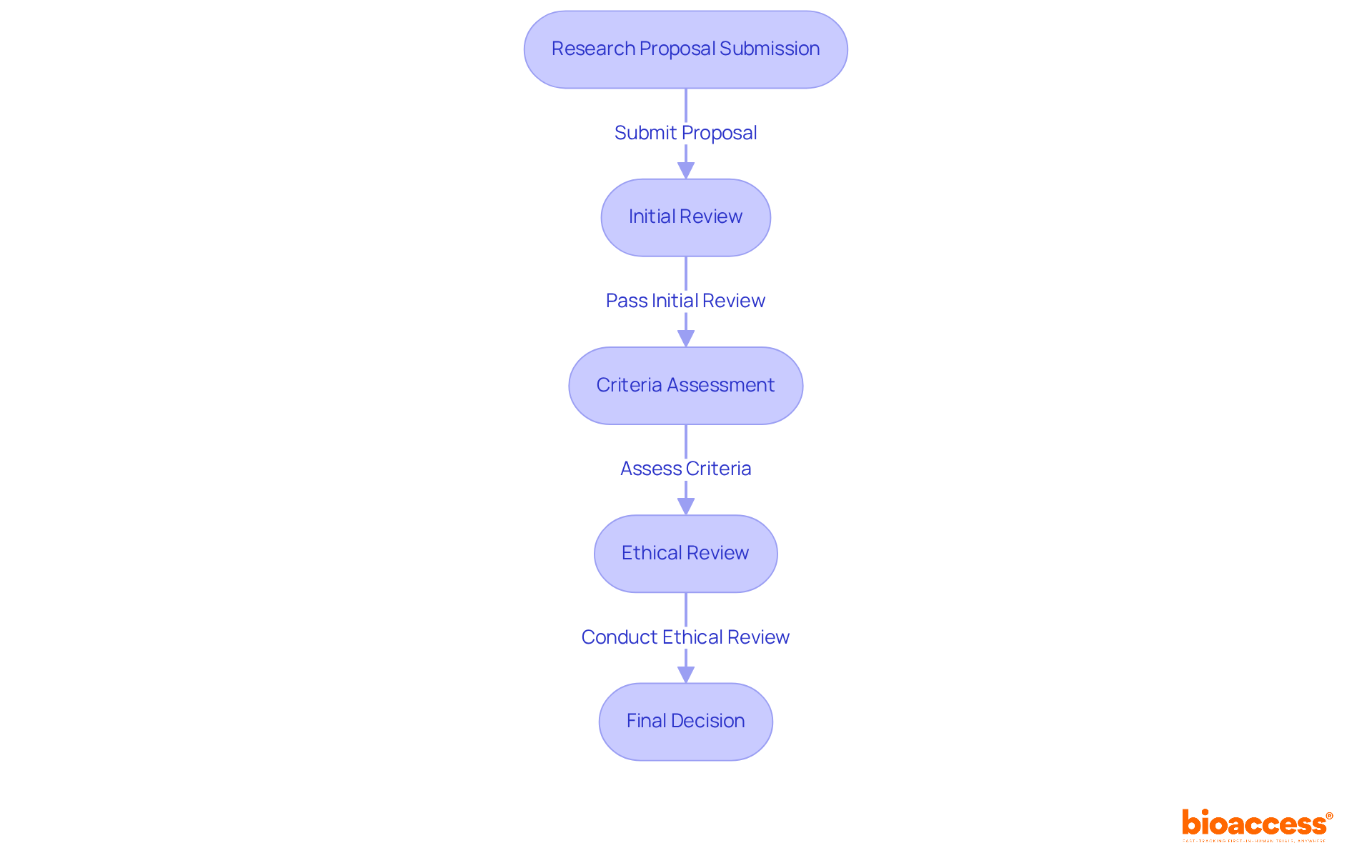
In clinical research, the ethics board SOPs in Bosnia and Herzegovina play a crucial role by clearly delineating the roles and responsibilities of each board member. They outline the processes for reviewing research proposals in accordance with the ethics board SOPs in Bosnia and Herzegovina, specifying the criteria for ethical review, the submission process, and the expected timeline for reviews. Research shows that organizations with effective SOPs can achieve a remarkable 60% reduction in compliance-related risks and a 31% improvement in organizational performance. This underscores the importance of clarity in these documents.
Utilizing a standardized format not only enhances readability but also ensures that all members are aligned. For instance, incorporating flowcharts or checklists can visually depict the evaluation process, facilitating adherence to established protocols. As Albert Schweitzer observed, 'A man is ethical only when life, as such, is sacred to him.' This statement highlights the necessity for ethics committees to function transparently and effectively, ensuring that ethical considerations remain at the forefront of clinical research.
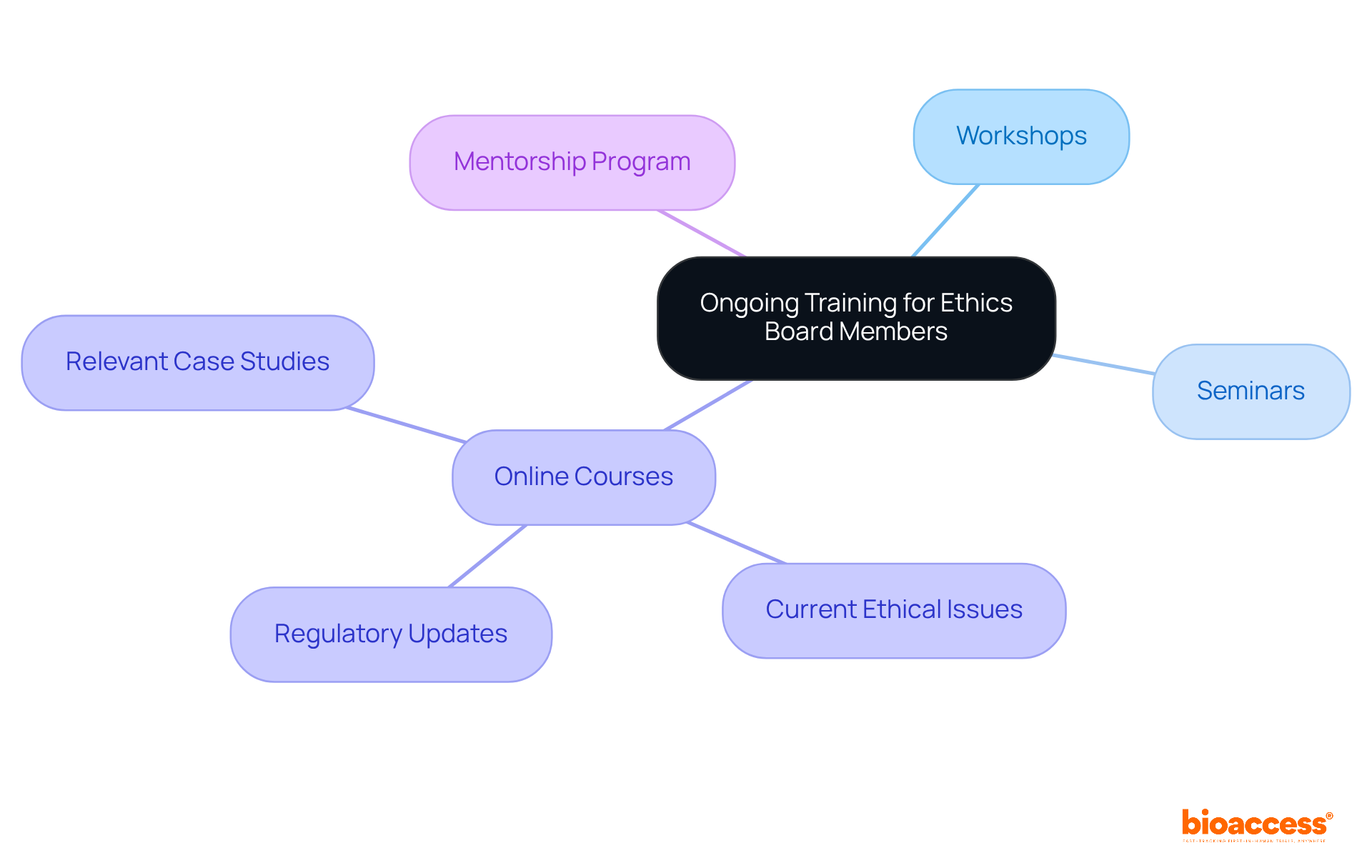
To ensure that ethics committee participants are thoroughly equipped to handle ethical evaluations, it is essential to implement continuous training programs. These programs should encompass:
Engaging with external experts in the field not only provides valuable insights but also fosters discussions on best practices. Furthermore, establishing a mentorship program where seasoned professionals assist newcomers can significantly enhance knowledge transfer and cultivate a cohesive team.
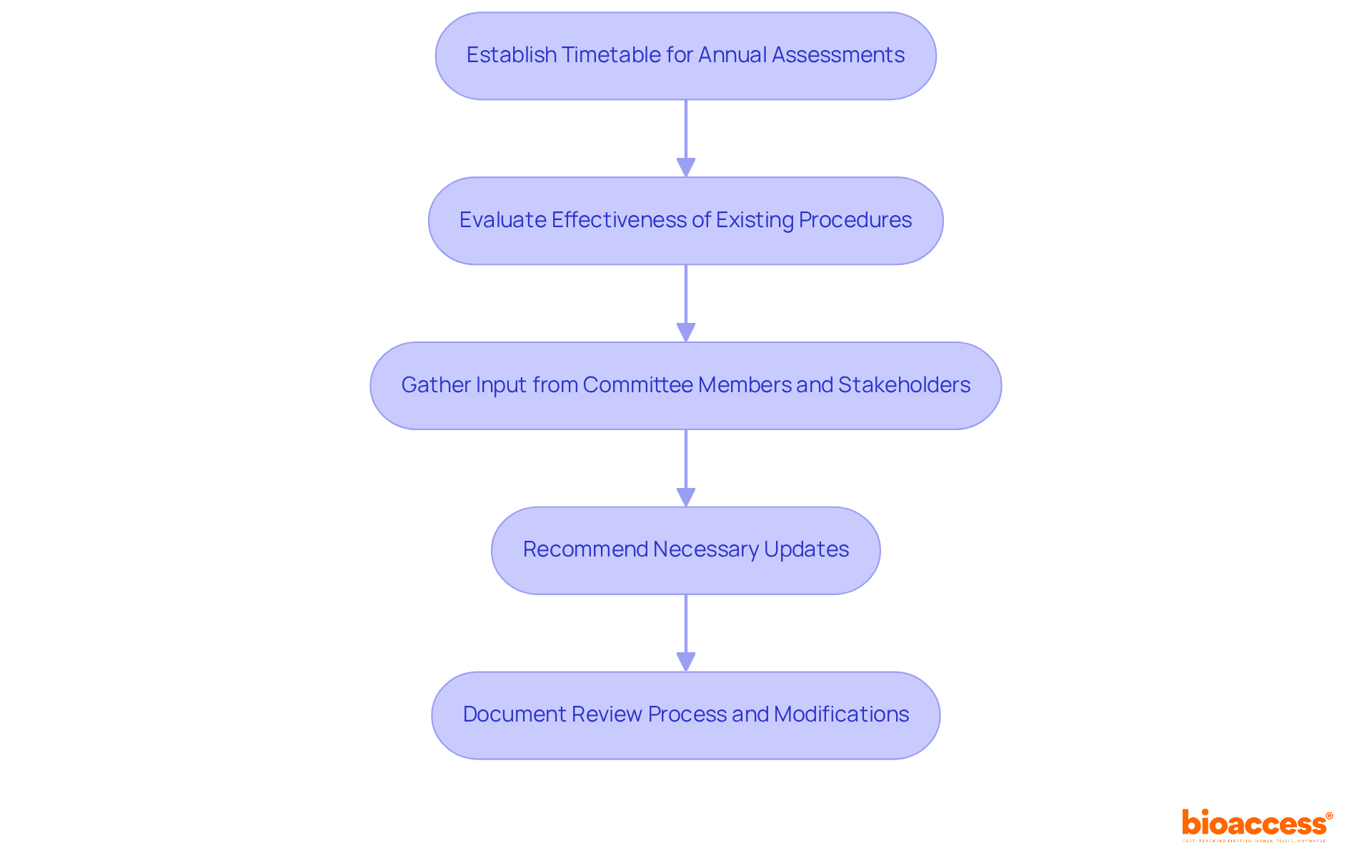
Establishing a systematic evaluation process for Standard Operating Procedures (SOPs) is essential in clinical research. This ensures that SOPs remain current and compliant with evolving practices and regulations. By creating a timetable for annual assessments, committee members can evaluate the effectiveness of existing procedures and recommend necessary updates. Research indicates that small businesses should review their critical SOPs every 6 to 12 months, with quarterly evaluations for high-risk or rapidly changing processes.
Gathering input from committee members and stakeholders during these evaluations is vital. It helps identify challenges and opportunities for improvement, fostering a culture of continuous advancement. Documenting the review process and any modifications made not only enhances transparency but also strengthens accountability within the ethics board SOPs in Bosnia and Herzegovina. For example, implementing an exception log for SOP deviations can improve communication among stakeholders and ensure ongoing enhancement.
This structured approach guarantees that SOPs are not static documents but dynamic tools that evolve with the clinical research landscape. As Gabriele Schmidt, Associate Director of Clinical Research, aptly states, "SOPs should be user-friendly, as short as possible, and as detailed as needed for clarity." This perspective reinforces the need for SOPs to be accessible while maintaining the necessary detail for effective implementation.
The effectiveness of ethics board standard operating procedures (SOPs) in Bosnia and Herzegovina is crucial for ensuring compliance with local regulations and ethical standards in clinical research. Understanding the regulatory landscape and implementing best practices allows ethics boards to enhance their operations, fostering trust and participation in clinical trials. A well-structured approach to SOPs clarifies roles and responsibilities while promoting a culture of ethical integrity, which is essential for advancing research efforts.
Key practices include:
Each of these elements contributes to a robust ethical framework that supports the rights and welfare of research participants while bolstering the overall performance of the ethics committees. Regular training and systematic evaluations ensure that SOPs remain relevant and effective, adapting to the ever-evolving landscape of clinical research.
Ultimately, the commitment to ethical oversight in Bosnia and Herzegovina transcends regulatory requirements; it is a moral imperative that enhances the credibility of clinical research. By prioritizing education, clarity, and continuous improvement, ethics boards can significantly impact the quality of research conducted in the region. The call to action is clear: invest in these best practices to cultivate a more ethical, transparent, and effective clinical research environment that benefits all stakeholders involved.
What is the role of the ethics board sops in Bosnia and Herzegovina regarding clinical research?
The ethics board sops in Bosnia and Herzegovina play a crucial role in navigating the regulatory landscape that governs clinical research, ensuring compliance with local regulations and international standards.
What regulations and standards govern clinical research in Bosnia and Herzegovina?
Clinical research in Bosnia and Herzegovina is governed by the Agency for Medicinal Products and Medical Devices (ALMBiH) and international standards such as the Declaration of Helsinki.
Why is compliance with these regulations important?
Compliance with these regulations is essential for protecting the rights and welfare of research participants.
What does the statistics indicate about clinical trials in Bosnia?
Statistics reveal that Bosnia has the lowest number of clinical trials in the Balkans, indicating a pressing need for robust ethical oversight.
How can ethical oversight enhance clinical research participation?
Robust ethical oversight is vital for enhancing participation and building trust in clinical research.
What measures should be taken to ensure compliance with regulations?
Regular training sessions and workshops must be prioritized by the ethics board sops to ensure that standard operating practices align with regulations.
What is the benefit of investing in education and training for ethics committees?
Investing in education and training can significantly improve the landscape of clinical trials, ultimately benefiting both researchers and participants.