


Navigating the complexities of clinical trial recruitment in Croatia presents both challenges and opportunities for researchers. The landscape is shaped by stringent regulatory requirements and the necessity for effective partnerships, making it essential to understand best practices for successful study initiation. How can researchers streamline their recruitment logistics while ensuring compliance and fostering community engagement in this rapidly evolving environment? This article explores four key strategies that can enhance subject recruitment logistics in Croatian clinical sites, ultimately leading to more effective and efficient clinical trials.
Navigating the regulatory landscape in Croatia is crucial for the successful execution of clinical studies. Researchers must familiarize themselves with the Ordinance on Clinical Studies and Good Clinical Practice (GCP) guidelines, which set the ethical and scientific standards for conducting studies. Key steps include:
Reviewing Local Legislation: Compliance with the Clinical Trials Regulation (EU) No 536/2014 is essential. This regulation mandates electronic submissions and streamlines processes across EU member states, aiming to enhance patient safety and improve study transparency. Sponsors are required to submit a single application for all EU countries through the Clinical Trials Information System (CTIS).
Engaging with Regulatory Bodies: Establishing communication with the Agency for Medicinal Products and Medical Devices (HALMED) is vital. This engagement clarifies requirements and expedites approvals. Regulatory experts emphasize that proactive communication can significantly reduce approval timelines, which are expected to be harmonized under the new regulation.
Documentation: Maintaining meticulous records of all regulatory submissions and approvals is essential for facilitating audits and inspections. Incomplete documentation can lead to considerable delays; in fact, previous cases showed that 28% of applications were postponed due to the need for additional information. Ensuring that all documentation is complete and readily accessible can mitigate risks associated with regulatory non-compliance.
By following these practices, researchers can enhance their chances of prompt study initiation and effectively manage the complexities of the regulatory environment.
Establishing strong collaborations with local healthcare providers is essential for effective subject recruitment logistics for Croatian clinical sites. These partnerships not only simplify hiring but also enhance the overall quality of the trial by ensuring individuals receive thorough care throughout the study.
Identify Key Stakeholders: Engage with physicians, clinics, and hospitals relevant to the trial's therapeutic area. Their knowledge can guide hiring strategies and improve the study's credibility.
Educational Initiatives: Conduct workshops and informational sessions to educate healthcare providers about the study's objectives, benefits, and participant eligibility criteria. This ensures they are well-informed advocates for the research.
Referral Networks: Establish organized referral channels that allow healthcare providers to suggest suitable patients. This approach enhances enrollment rates and promotes community engagement.
By fostering these relationships, you not only streamline the subject recruitment logistics for Croatian clinical sites but also build a foundation of trust and expertise that is vital for the success of clinical trials.
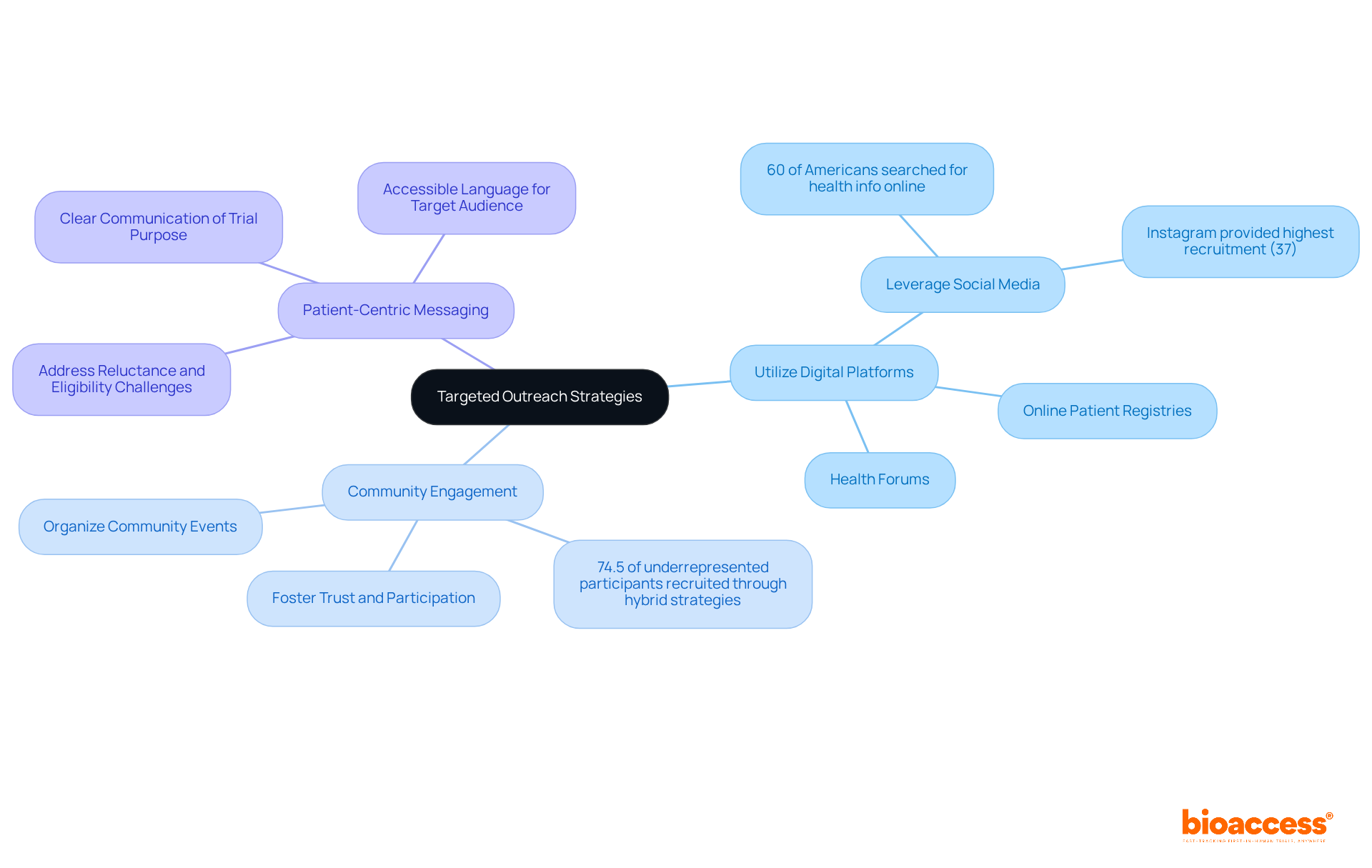
Effective hiring is crucial in clinical research, especially for Medtech, Biopharma, and Radiopharma startups facing unique challenges. To enhance recruitment efforts, consider these focused outreach strategies:
By employing these strategies, researchers can enhance visibility and attract a diverse group of participants, ultimately improving study outcomes. Given that dropout rates in clinical trials average 30%, these approaches are essential for improving retention and engagement.
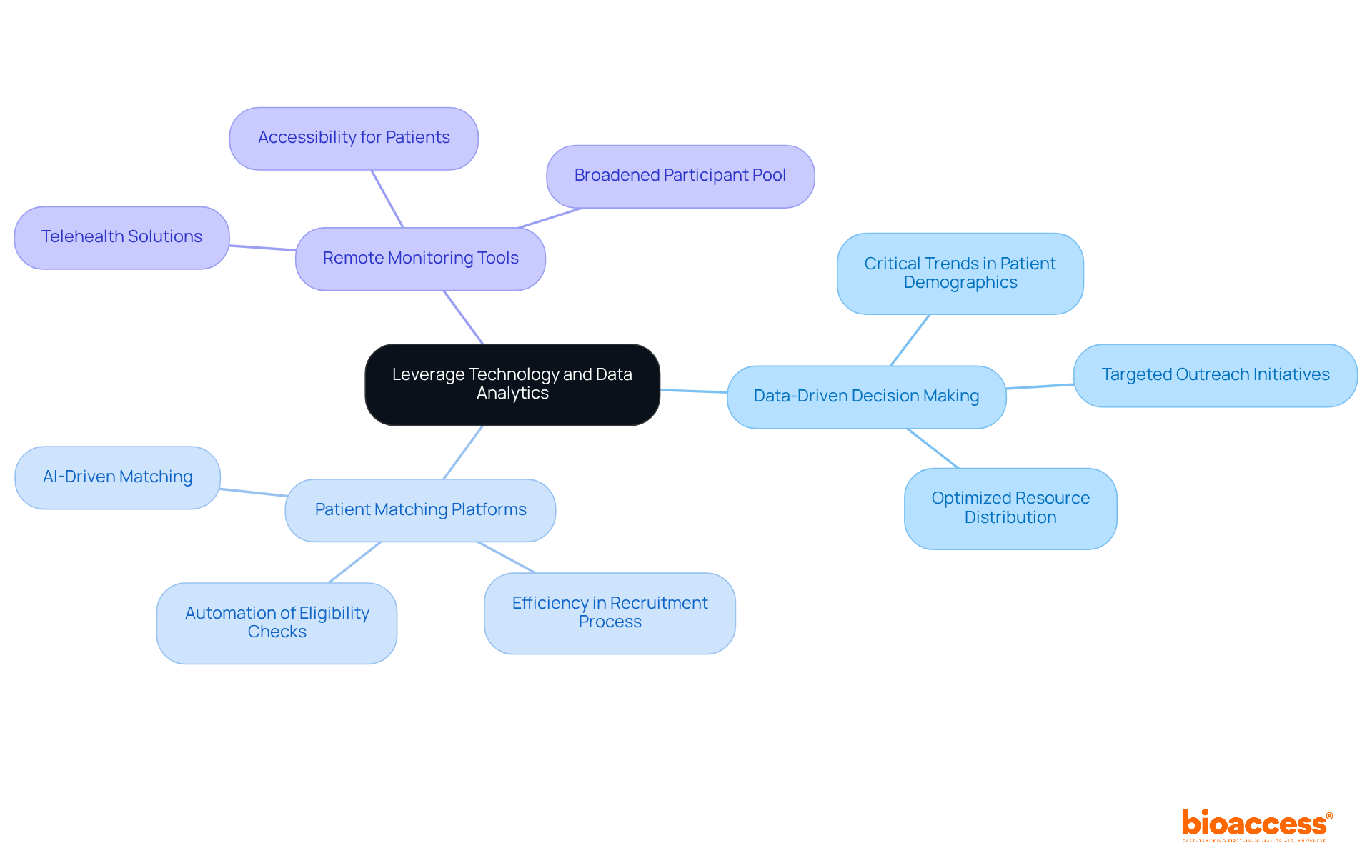
Incorporating technology and data analytics into subject recruitment logistics for Croatian clinical sites is not just beneficial; it’s essential for enhancing both efficiency and effectiveness in clinical research. By leveraging these advancements, organizations can significantly improve their strategies for subject recruitment logistics for Croatian clinical sites and enhance participant experiences.
Data-Driven Decision Making: Analytics can reveal critical trends in patient demographics and recruitment patterns. This insight allows for targeted outreach initiatives and optimized resource distribution in the subject recruitment logistics for Croatian clinical sites, ensuring that hiring strategies meet the specific needs of prospective participants. Are you currently utilizing data to inform your hiring decisions?
Patient Matching Platforms: The implementation of AI-driven platforms can revolutionize the recruitment process. By efficiently matching potential participants with trials based on their medical history and eligibility criteria, these tools enhance the subject recruitment logistics for Croatian clinical sites, saving time and effort. Imagine the impact on your recruitment rates if you could automate this process.
Remote Monitoring Tools: Telehealth solutions facilitate remote consultations and screenings, making participation more accessible for patients who face logistical barriers. This approach not only broadens the pool of potential participants but also enhances the subject recruitment logistics for Croatian clinical sites, improving the overall experience for those involved. How can you leverage technology to improve accessibility in your trials?
By harnessing these technological advancements, researchers can enhance subject recruitment logistics for Croatian clinical sites, elevate recruitment rates, reduce costs, and improve the participant experience. This ultimately leads to more successful clinical trials, underscoring the importance of embracing innovation in the Medtech landscape.
Navigating the complexities of subject recruitment logistics in Croatian clinical sites is not just important; it’s essential for the success of clinical trials. Understanding local regulatory requirements, cultivating partnerships with healthcare providers, implementing targeted outreach strategies, and leveraging technology are all critical steps researchers must take to enhance their recruitment efforts and streamline the trial process.
Key insights reveal the necessity of compliance with Croatian regulations, the value of building strong relationships with local healthcare professionals, and the effectiveness of utilizing digital platforms for outreach. Moreover, incorporating data analytics and technology not only boosts recruitment efficiency but also enhances the overall participant experience, ultimately leading to more successful clinical outcomes.
As the landscape of clinical research evolves, embracing these best practices becomes vital for researchers aiming to improve subject recruitment logistics. By prioritizing collaboration, innovation, and strategic outreach, stakeholders can meet the needs of their trials while contributing to the broader advancement of medical research in Croatia. Engaging with these strategies today can pave the way for more effective and inclusive clinical trials in the future.
What is the importance of understanding local regulatory requirements in Croatia for clinical studies?
Understanding local regulatory requirements is crucial for the successful execution of clinical studies in Croatia, as it ensures compliance with ethical and scientific standards.
What regulations must researchers comply with when conducting clinical studies in Croatia?
Researchers must comply with the Clinical Trials Regulation (EU) No 536/2014, which mandates electronic submissions and aims to enhance patient safety and improve study transparency.
How should sponsors submit applications for clinical trials in Croatia?
Sponsors are required to submit a single application for all EU countries through the Clinical Trials Information System (CTIS).
Why is it important to engage with regulatory bodies like HALMED?
Engaging with the Agency for Medicinal Products and Medical Devices (HALMED) is vital for clarifying requirements and expediting approvals, which can significantly reduce approval timelines.
What role does documentation play in the regulatory process for clinical studies?
Maintaining meticulous records of all regulatory submissions and approvals is essential for facilitating audits and inspections. Incomplete documentation can lead to delays in the approval process.
What can happen if documentation is incomplete during the regulatory submission process?
Incomplete documentation can result in considerable delays, with previous cases showing that 28% of applications were postponed due to the need for additional information.
How can researchers enhance their chances of prompt study initiation?
By following regulatory practices such as engaging with regulatory bodies and ensuring complete documentation, researchers can enhance their chances of prompt study initiation and manage regulatory complexities effectively.