

Creating a trial operations dashboard for Australian studies represents a pivotal advancement in clinical research efficiency. This tool streamlines patient enrollment and site performance monitoring, enhancing operational oversight and equipping stakeholders with actionable insights. Yet, a significant challenge remains: how can researchers effectively define the dashboard's purpose and integrate the right metrics and data sources? It's crucial to consider how this dashboard can meet the diverse needs of research teams while driving impactful decision-making.
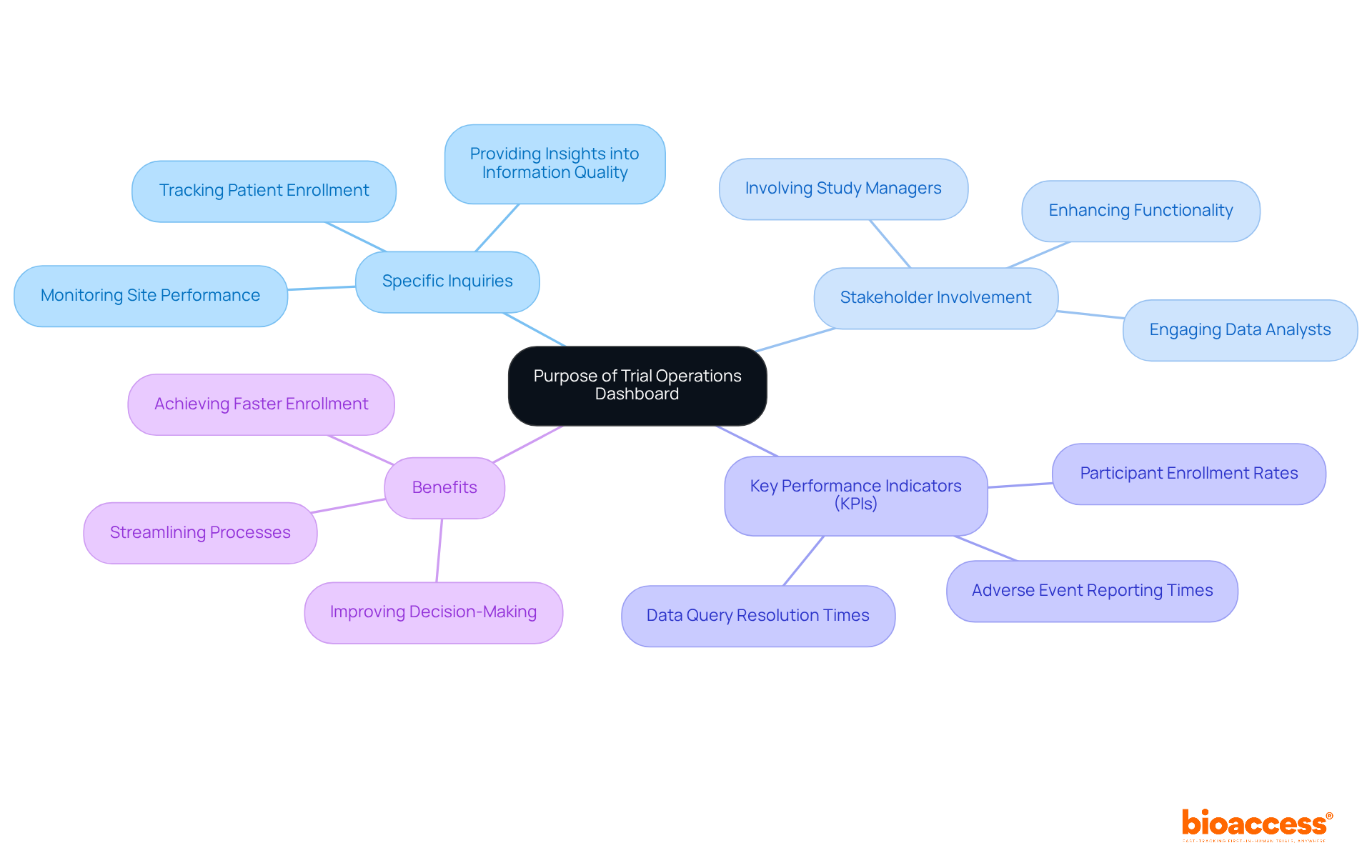
Creating a trial operations dashboard for Australian studies requires a clear definition of its purpose. What specific inquiries should the interface address? Consider how creating a trial operations dashboard for Australian studies can assist in tracking patient enrollment, monitoring site performance, or providing insights into information quality. Documenting these objectives is essential, as it will guide the development process. Involving key stakeholders, such as study managers and data analysts, is important for creating a trial operations dashboard for Australian studies that meets their needs and expectations. This collaborative approach not only enhances functionality but also increases the tool's value to its users, ultimately improving decision-making in research studies, such as creating a trial operations dashboard for Australian studies.
Moreover, establishing Key Performance Indicators (KPIs) is essential when creating a trial operations dashboard for Australian studies, as it provides measurable objectives that enhance its effectiveness. Input from clinical study managers offers a practical perspective on these goals, ensuring the interface fulfills its intended function. With bioaccess®'s capabilities, including study setup and project management, you can achieve patient enrollment 50% faster and save $25K per patient. Leverage FDA-ready information to streamline your processes and effectively tackle recruitment challenges.
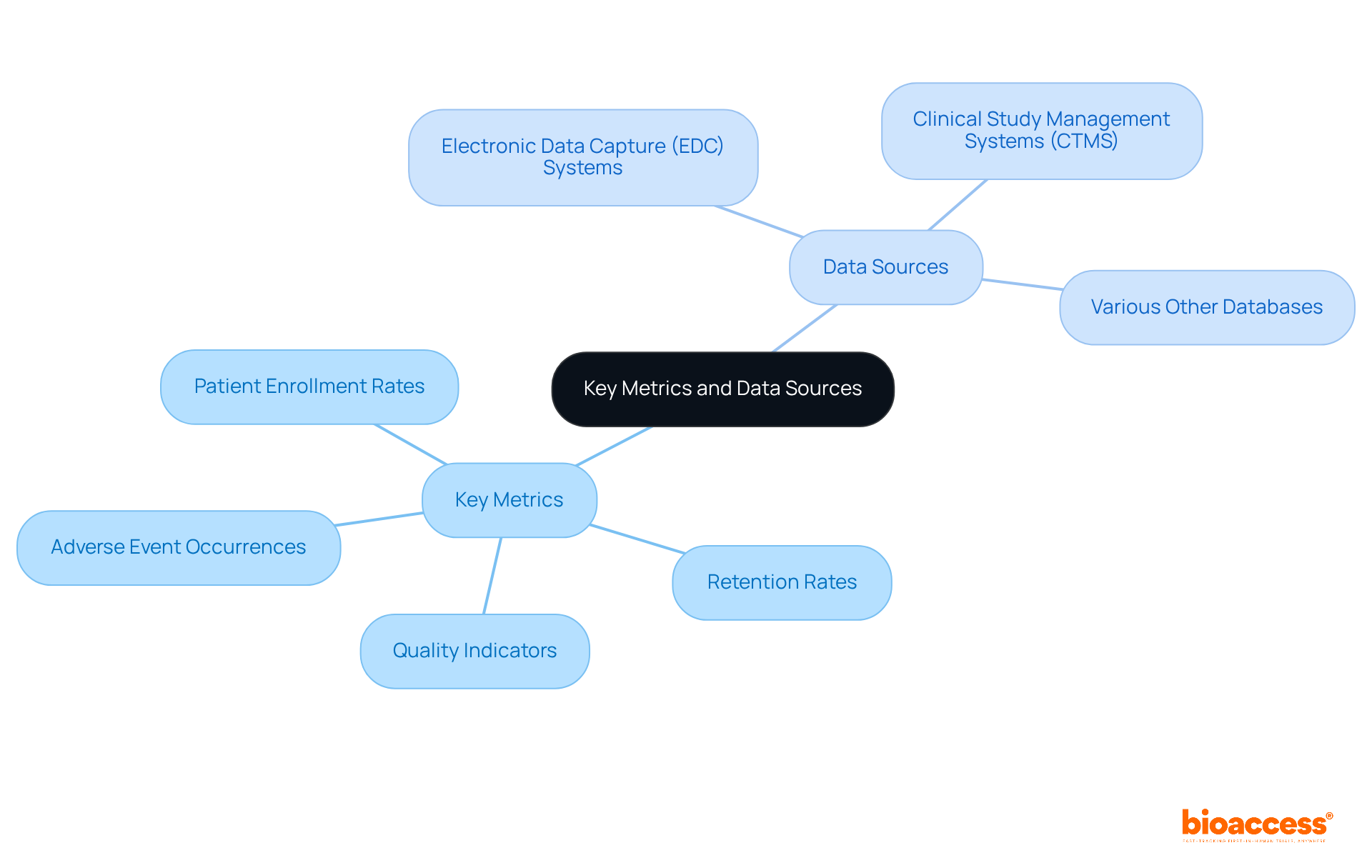
Start by pinpointing the essential metrics to feature on your dashboard. In clinical research, key metrics typically encompass:
After compiling this list, the next step is to identify the sources that will provide this critical information. Common sources include:
It's vital to ensure these data sources are reliable and that you have the necessary access to the data. For instance, research shows that patient enrollment rates can vary significantly based on study design and sponsorship, with industry-backed studies achieving higher median enrollment rates compared to those funded by the government. Documenting these metrics along with their sources will create a clear reference for the development process, facilitating effective monitoring and informed decision-making throughout the study's lifecycle.
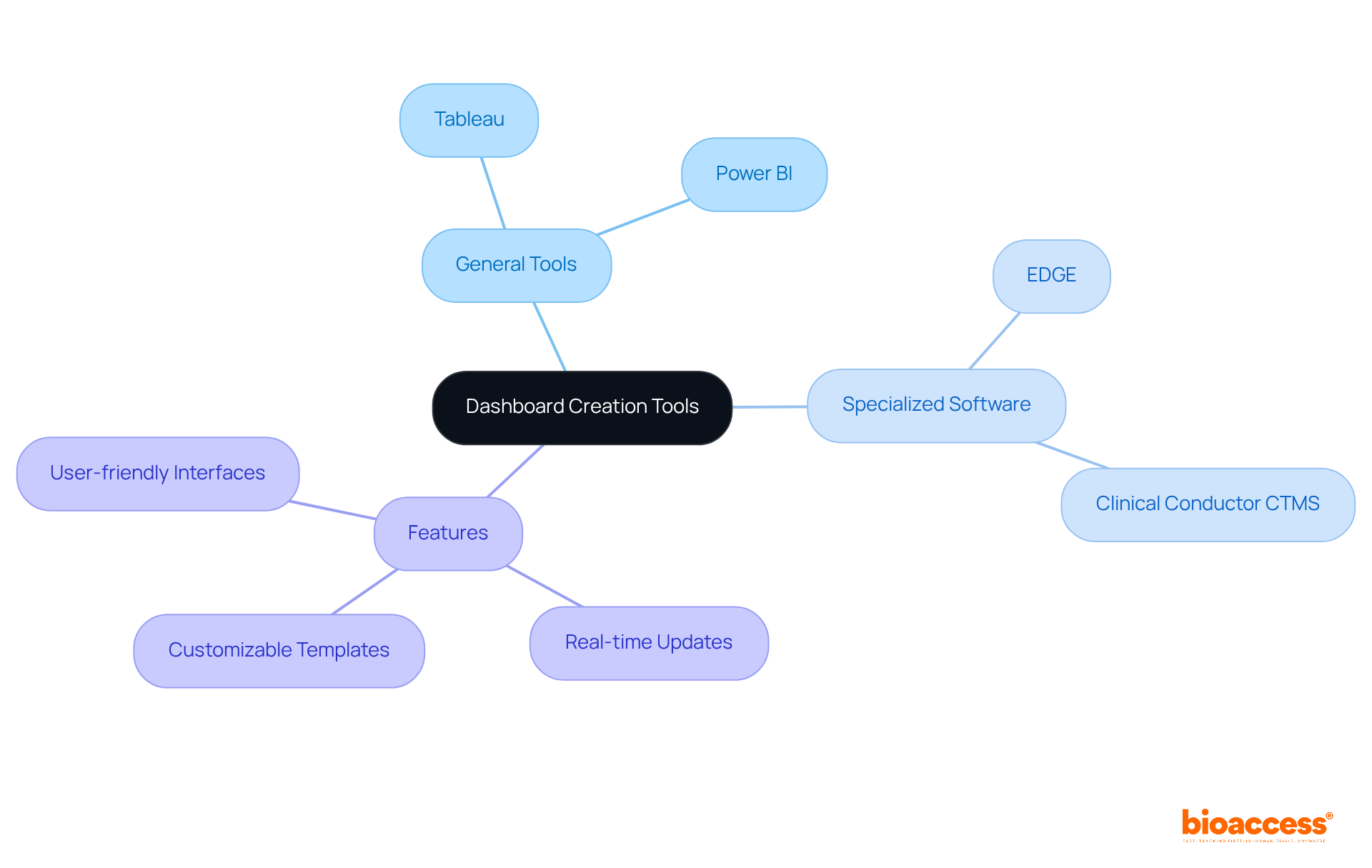
With your metrics and information sources recognized, it’s crucial to select the right tools and software for constructing your display. Consider options like:
Assess each tool based on its ability to connect with your information sources, ease of use, and visualization options. Look for features such as:
For instance, Power BI excels in integrating operational and clinical information, enabling comprehensive tracking of trial metrics. Additionally, specialized platforms like EDGE provide real-time information access, while Clinical Conductor CTMS is preferred by research sites for its integrated compliance tracking and reporting capabilities. Familiarizing yourself with the functionalities of your chosen tool is essential for maximizing its potential in your interface design.
By leveraging the appropriate software, you can create a dynamic interface that enhances information visibility and supports informed decision-making throughout the experimental process.
When designing the layout and user interface of your clinical trial display, it’s essential to start with a wireframe that clearly outlines where each metric will be displayed. Prioritize key metrics by positioning them prominently, especially in the upper-left section, as research shows that users typically scan information in an F-shaped pattern. Incorporate visual elements like graphs, charts, and color coding to enhance readability and engagement; effective data visualization significantly boosts user comprehension. Additionally, utilize white space effectively to improve readability and design balance, steering clear of unnecessary decorations to maintain simplicity.
An intuitive design is crucial, allowing users to navigate effortlessly between various sections of the interface. Integrating user feedback during the design process is vital; studies indicate that interfaces tailored to user preferences lead to greater satisfaction and usability. Consistent feedback from users can enhance interface designs to better meet their needs. Creating a trial operations dashboard for Australian studies facilitates prompt decision-making and improves overall operational effectiveness. By focusing on these design principles, you can create a display that is both efficient and user-friendly, ultimately supporting the success of your research initiatives in healthcare, particularly in creating a trial operations dashboard for Australian studies.
Begin by integrating recognized information sources into your clinical study interface. Follow the specific instructions provided by your chosen dashboard software to establish connections with each information source, ensuring a seamless flow of details. This integration is crucial for enabling real-time updates, which are essential for effective trial management. Testing the integration is vital; confirm that the data is accurate and displayed correctly to maintain data integrity.
Once the integration is complete, implement the control panel within your organization. Ensure that relevant stakeholders have access to the interface, facilitating collaboration and informed decision-making. To maximize the dashboard's utility, conduct training sessions that equip users with the skills to navigate and utilize the dashboard effectively. This approach not only boosts user involvement but also fosters a culture of data-informed decision-making within your research team.
Statistics reveal that organizations leveraging real-time information updates in research management can significantly enhance operational efficiency and reduce delays. For instance, the trial information management service market is projected to grow at a CAGR of 8.3% from 2025 to 2035, underscoring the increasing demand for advanced visualization tools. Successful implementations in clinical research organizations demonstrate that interactive displays can streamline processes, enhance data interpretation, and ultimately lead to improved patient outcomes.
To ensure your interface operates at peak efficiency, it's crucial to conduct comprehensive testing with a diverse group of users. Encourage them to engage with the interface and provide insights on its functionality and usability. Focus on key areas such as loading times, data accuracy, and overall user experience. Notably, studies indicate that practices with higher engagement levels on visual displays tend to perform better on quality measures. In fact, 28 studies have reported a reduction in length of stay (LOS), underscoring how user feedback can significantly enhance effectiveness.
Utilize structured feedback mechanisms, such as surveys and focus groups, to gather valuable insights. This approach not only helps identify usability issues but also allows for prioritizing features that align with user needs. A systematic review has emphasized that visual displays customized according to user feedback can lead to better clinical outcomes, including increased patient satisfaction and reduced expenses. Remarkably, 29 findings have shown cost reductions linked to effective display design.
Based on the feedback gathered, make necessary adjustments to enhance the system's performance. This may involve refining the layout for better navigation, enhancing data visualizations for clearer insights, or improving data integration processes to ensure real-time updates. As Enrico Coiera observed, "Digital interfaces are commonly utilized in numerous hospital environments and activities, and this thorough systematic review offers evidence that, when an intervention is executed effectively, notable enhancements in essential medical, process, and financial results are achievable." Once you have addressed the feedback and are confident in the dashboard's performance, proceed with its official launch for creating a trial operations dashboard for Australian studies, ensuring that it meets the needs of all stakeholders involved.

Creating a trial operations dashboard for Australian studies is a complex task that requires clear objectives and strategic planning. This guide has outlined how to effectively build such a dashboard, highlighting the critical role of collaboration with stakeholders, the identification of key metrics, and the selection of appropriate tools. By concentrating on these elements, researchers can significantly enhance their decision-making processes and operational efficiency.
Key insights include the necessity of establishing a clear purpose for the dashboard, selecting relevant metrics and data sources, and ensuring a user-friendly design. Integrating reliable data sources and conducting iterative testing of the dashboard further solidify its functionality and effectiveness. By adhering to these steps, organizations can markedly improve their trial management processes, leading to better patient outcomes and operational success.
Ultimately, creating a trial operations dashboard transcends mere data visualization; it fosters a culture of informed decision-making in clinical research. Embracing these best practices empowers researchers to leverage data more effectively, streamline operations, and contribute to advancements in healthcare. Engaging in this process is essential for any organization aiming to enhance its research capabilities and drive impactful results in the field of Australian studies.
What is the purpose of a trial operations dashboard in Australian studies?
The purpose of a trial operations dashboard in Australian studies is to track patient enrollment, monitor site performance, and provide insights into information quality. Defining these objectives is essential as it guides the development process.
Who should be involved in creating a trial operations dashboard?
Key stakeholders such as study managers and data analysts should be involved in creating a trial operations dashboard to ensure it meets their needs and expectations. This collaborative approach enhances functionality and increases the tool's value.
Why are Key Performance Indicators (KPIs) important for a trial operations dashboard?
KPIs are important because they provide measurable objectives that enhance the effectiveness of the dashboard. Input from clinical study managers helps ensure that the interface fulfills its intended function.
What are some key metrics to include on a trial operations dashboard?
Key metrics typically include patient enrollment rates, retention rates, adverse event occurrences, and quality indicators.
What are common data sources for a trial operations dashboard?
Common data sources include electronic data capture (EDC) systems, clinical study management systems (CTMS), and various other databases.
Why is it important to ensure the reliability of data sources?
It is important to ensure the reliability of data sources to guarantee accurate information for monitoring and decision-making throughout the study's lifecycle.
How can study design and sponsorship affect patient enrollment rates?
Research indicates that patient enrollment rates can vary significantly based on study design and sponsorship, with industry-backed studies typically achieving higher median enrollment rates compared to those funded by the government.