


In the fast-paced realm of clinical research, the speed and efficiency of review cycles can significantly impact timely patient access to innovative therapies, potentially leading to prolonged delays. As organizations navigate the complexities of regulatory processes, grasping the key differences between the European Medicines Agency (EMA) and Big Data Analytics (BDA) review cycles is essential. This article explores the distinct methodologies employed by bioaccess®, EMA, and BDA, shedding light on how these approaches influence timelines, ethical considerations, and overall effectiveness in bringing new treatments to market. With the stakes high, stakeholders must consider:
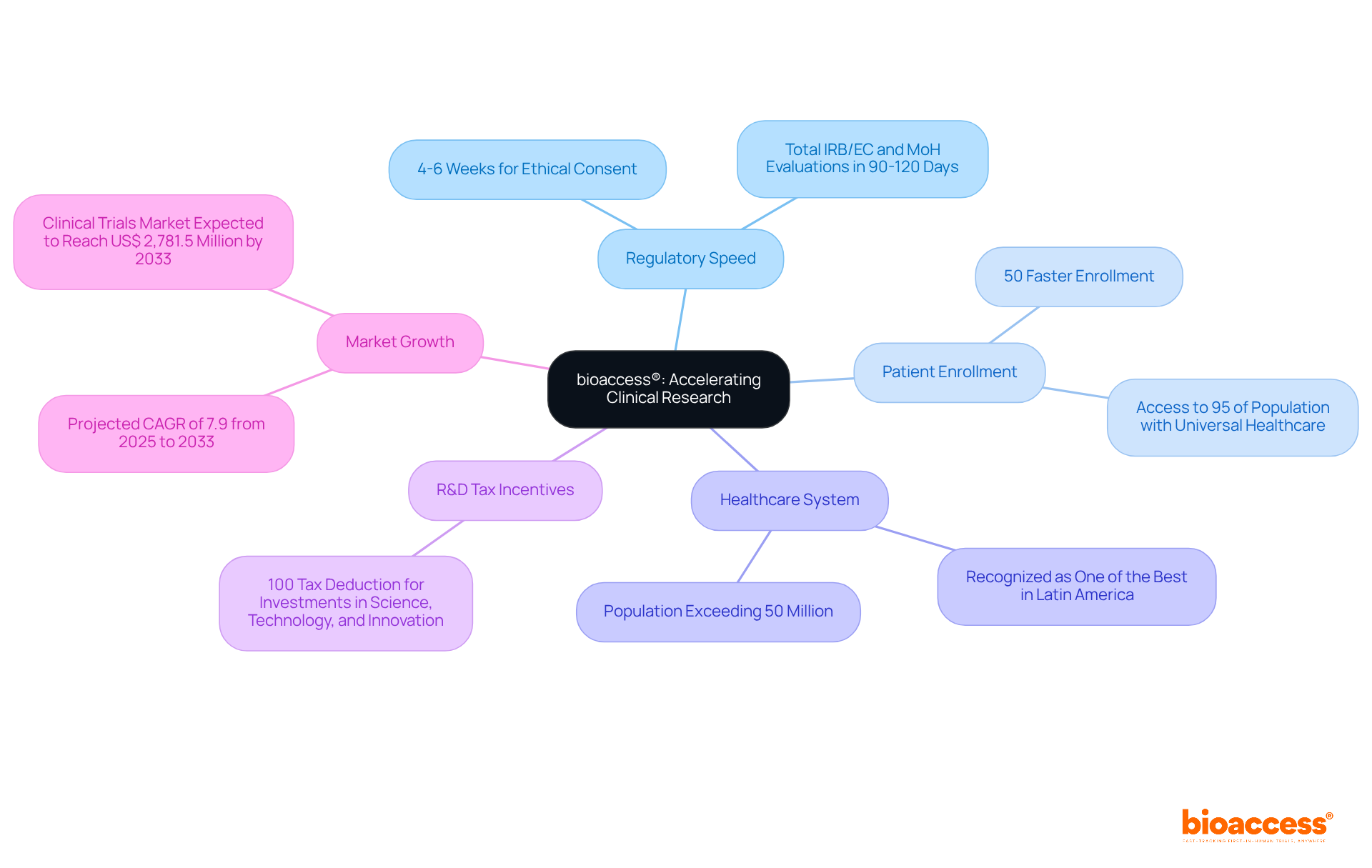
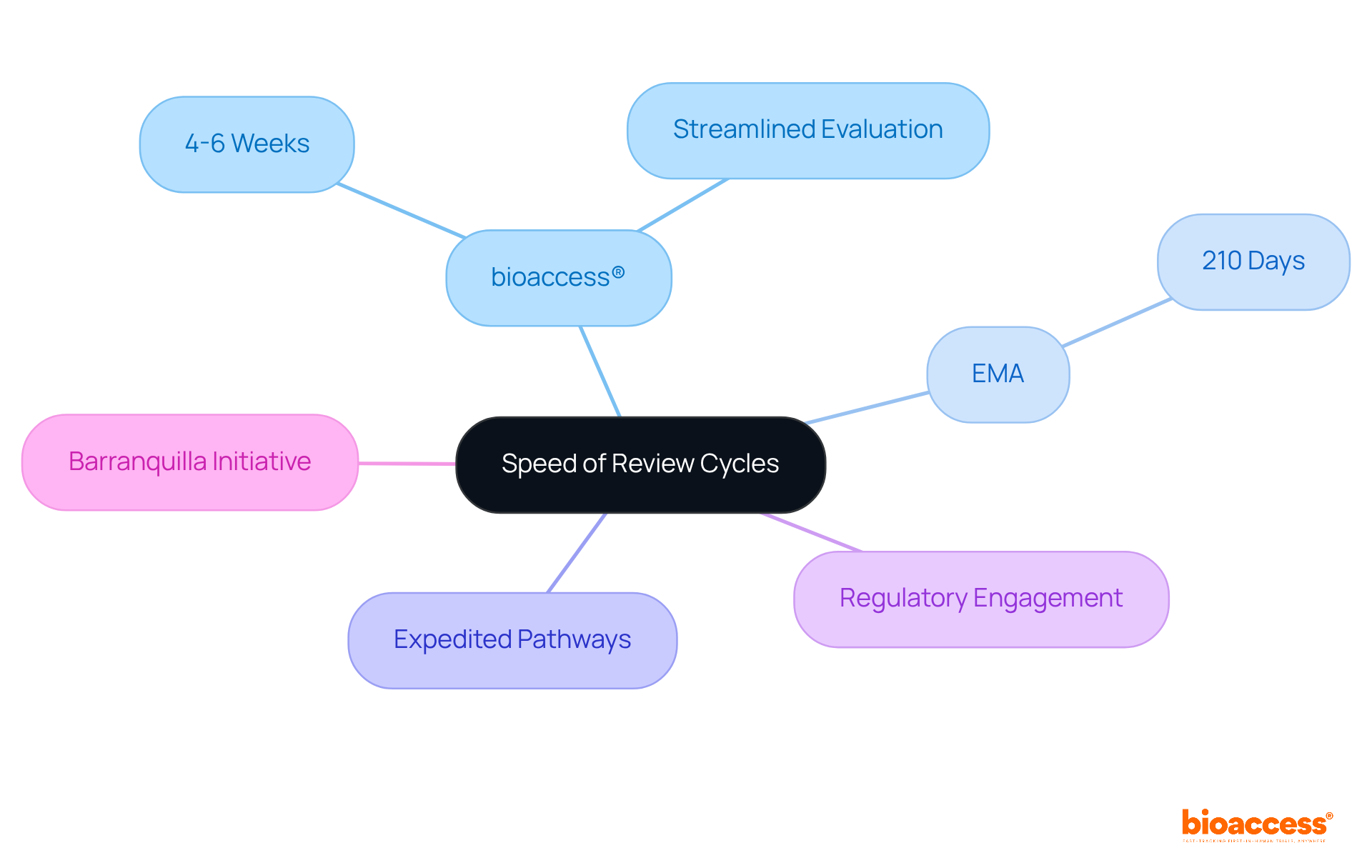
bioaccess® leverages the regulatory speed of Latin America, particularly Colombia, to significantly shorten clinical research review cycles. By employing efficient methods and regional expertise, the organization achieves ethical consent in an impressive 4-6 weeks-substantially faster than the typical durations seen in conventional markets, which can extend for several months. This agility not only accelerates the path to market for Medtech, Biopharma, and Radiopharma innovators but also enhances their competitive edge on a global scale.
For instance, bioaccess® can secure patient enrollment 50% faster than traditional sites, allowing firms focused on cutting-edge treatments to navigate the validation process in record time. Colombia's healthcare system, recognized as one of the best in Latin America, bolsters this efficiency, with a population exceeding 50 million and universal healthcare coverage that facilitates robust patient recruitment. Furthermore, the region provides substantial R&D tax incentives, including a 100% tax deduction for investments in science, technology, and innovation projects, making it an appealing destination for clinical research.
As the Latin American clinical trials market is projected to grow significantly, with a compound annual growth rate of 7.9% from 2025 to 2033, the region's regulatory efficiency positions it as a prime location for clinical research. Ethical endorsements are crucial for maintaining public trust and ensuring the safety and effectiveness of new medical technologies. By prioritizing ethical approvals and minimizing assessment cycles, bioaccess® empowers its clients to accelerate their product development and commercialization efforts effectively.
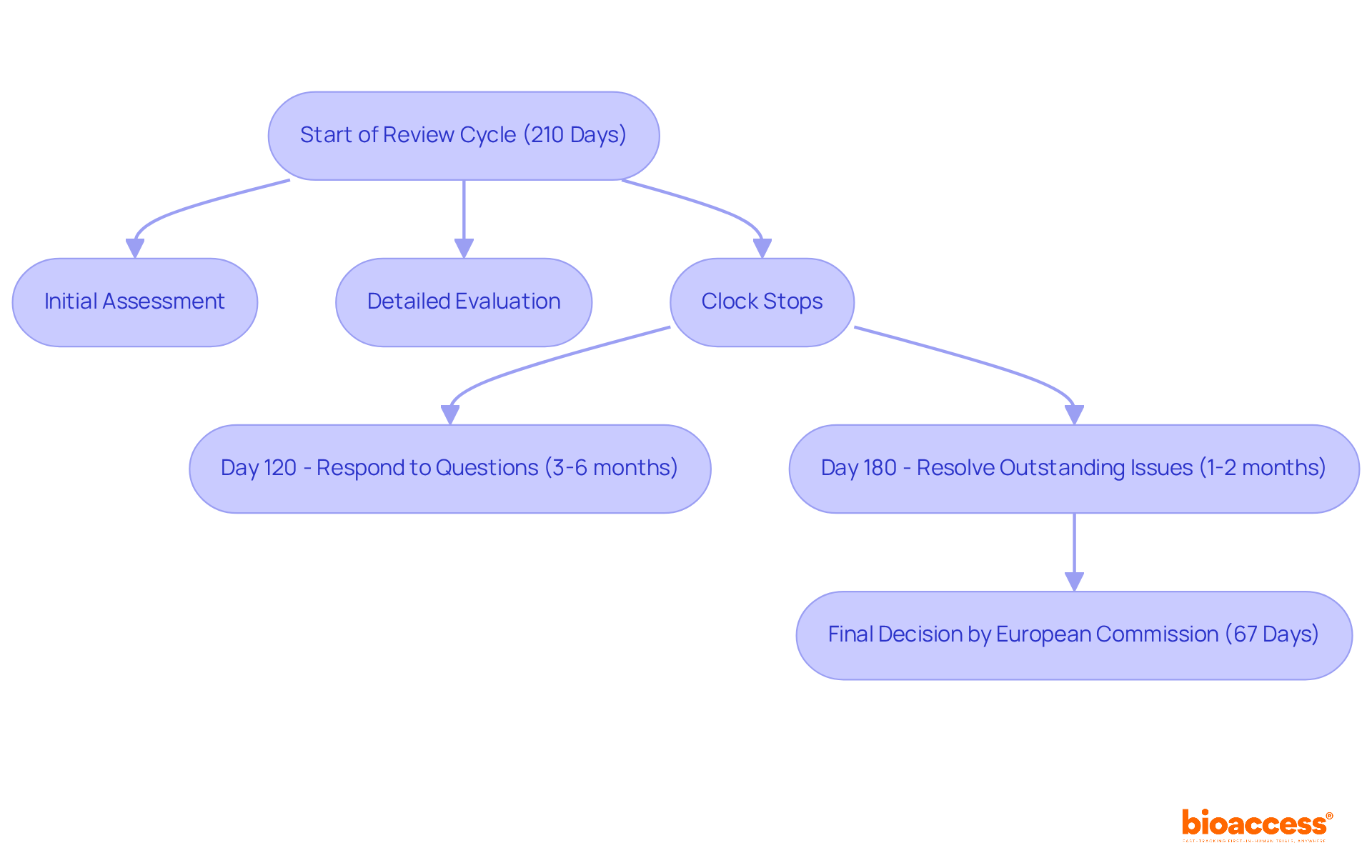
The European Medicines Agency (EMA) plays a crucial role in ensuring the safety and effectiveness of medications through a meticulously structured evaluation cycle for standard applications, typically lasting 210 days. This comprehensive timeline includes several critical stages:
Significantly, the EMA's stringent criteria mandate that all medications meet rigorous safety and effectiveness standards. While this guarantees high-quality results, it can lead to longer assessment periods compared to more accelerated regulatory environments. For instance, the EMA's classic procedure incorporates two significant clock stops:
In 2023, only 35% of marketing authorization applications (MAAs) were submitted on time, underscoring the complexities involved in navigating this procedure. The EMA's commitment to thorough evaluations is further highlighted by the average of 67 days the European Commission takes to make a final decision following a positive opinion from the EMA. This emphasizes the importance of meticulous preparation and adherence to timelines for successful marketing authorization.
Big Data Analytics (BDA) is revolutionizing regulatory assessment cycles in clinical research. By leveraging extensive datasets, regulatory agencies can identify trends, streamline workflows, and significantly enhance decision-making efficiency. For instance, organizations that have integrated BDA into their review processes report authorization times that are up to 50% faster than traditional methods. This acceleration is vital, particularly as the average research and development cycle now exceeds 15 years, with Phase III trial durations increasing by 47% over the past two decades.
The integration of BDA not only expedites the evaluation of clinical trial data but also contributes to improved patient outcomes. A prime example is bioaccess® with its innovative 'Patient Velocity' approach, which achieves 50% faster patient enrollment for cardiology and neurology cohorts, alongside savings of $25K per patient by providing FDA-ready data that eliminates rework and delays. The impact of BDA on regulatory endorsements is profound, as agencies increasingly rely on data-driven insights to inform their decisions. Statistics indicate that employing BDA in regulatory assessment activities has led to enhanced efficiency, with predictive analytics models achieving up to 85% accuracy in forecasting trial outcomes. This data-centric approach is crucial for navigating the complexities of modern clinical trials and ensuring that innovative therapies reach patients more swiftly.
Moreover, partnerships like that of bioaccess with GlobalCare Clinical Trials in Colombia illustrate the effectiveness of these methodologies, resulting in over a 50% reduction in recruitment time and a retention rate exceeding 95%. As the landscape of clinical research evolves, the collaboration between organizations and the strategic use of BDA will be essential in overcoming challenges and driving progress.

In the realm of clinical trial evaluation cycles, bioaccess® stands out by securing ethical approvals in just 4-6 weeks. This is a striking contrast to the EMA's standard assessment period of approximately 210 days. Such a significant difference underscores the advantages of conducting clinical trials in regions with more adaptable regulatory frameworks.
With over 20 years of experience in Medtech and real-time data analysis, bioaccess® effectively streamlines evaluation times and enhances clinical trial efficiency. Statistics indicate that expedited pathways, like those provided by bioaccess®, can significantly reduce time to market, enabling innovative therapies to reach patients more quickly. Experts emphasize that engaging with regulatory bodies early in the development process not only boosts submission success rates but also accelerates trial timelines, benefiting both researchers and patients alike.
Moreover, the partnership between bioaccess® and Caribbean Health Group aims to establish Barranquilla as a premier destination for clinical trials in Latin America. This initiative, backed by Colombia's Minister of Health, further elevates the region's attractiveness for clinical research. As the landscape of clinical trials evolves, collaboration and strategic positioning will be crucial for advancing healthcare solutions.
Ethical considerations are fundamental in the examination cycles of clinical research, significantly influencing both timelines and outcomes. bioaccess® stands out by emphasizing ethical compliance, ensuring that all studies adhere to local regulations and international standards. This commitment fosters trust and integrity in research, which is essential for all stakeholders involved. In contrast, the European Medicines Agency (EMA) incorporates rigorous ethical evaluations within its assessment processes, which illustrates the differences between EMA and BDA review cycles. This thoroughness often extends timelines, reflecting the critical importance of participant safety and data integrity. Such an emphasis on ethics not only safeguards participants but also enhances the credibility of research findings.
Moreover, the methodologies employed by the BDA illustrate the differences between EMA and BDA review cycles, integrating ethical frameworks that underscore the universal importance of ethics in clinical trials. By navigating these complexities, bioaccess® effectively streamlines ethical compliance, ensuring that studies are conducted efficiently while upholding high ethical standards. This approach not only addresses key challenges in the Medtech landscape but also reinforces the necessity for collaboration among all parties involved in clinical research.
Recent regulatory reforms significantly impact evaluation cycles for bioaccess®, highlighting the differences between EMA and BDA review cycles and underscoring their relevance in clinical research. For bioaccess®, these modifications are poised to enhance its already swift authorization processes, typically finalizing ethical consent in just 4-6 weeks. In contrast, the differences between EMA and BDA review cycles may pose challenges for the EMA in adapting to these new regulations, potentially leading to extended evaluation times. Statistics reveal that the FDA's recent changes have notably reduced median review durations, with expedited routes like priority review and accelerated pathways being utilized more frequently. For instance, over half of new drug approvals now leverage at least one expedited designation, reflecting a broader trend towards faster approvals.
BDA plays a crucial role in assisting organizations as they navigate the differences between EMA and BDA review cycles in these evolving regulatory landscapes. By providing data-driven insights that align with the latest requirements, BDA ensures that companies can adapt swiftly and maintain compliance. This collaboration is essential for organizations aiming to thrive in the dynamic Medtech landscape. As the regulatory environment continues to evolve, the importance of leveraging expertise and insights cannot be overstated. Companies must act decisively to stay ahead of the curve and ensure their processes align with the latest standards.

bioaccess® emphasizes the critical role of stakeholder engagement in clinical research by fostering strong relationships with local regulatory bodies, ethics committees, and patient advocacy groups. This collaborative strategy not only enhances adaptability but also emphasizes the differences between EMA and BDA review cycles, as the European Medicines Agency (EMA) often employs more rigid engagement methods that can hinder flexibility. By utilizing real-time data and insights, the methodologies employed by bioaccess® significantly improve stakeholder interactions, promoting dynamic communication throughout the evaluation cycle.
This proactive engagement streamlines the regulatory assessment process, fostering a culture of transparency and trust. As a result, clinical trials benefit from faster and more efficient outcomes. In a landscape where collaboration is paramount, bioaccess® stands out by addressing key challenges in Medtech, ensuring that all stakeholders are aligned and informed. This approach not only builds credibility but also generates a desire for their services, reinforcing the conviction that effective engagement leads to superior results.

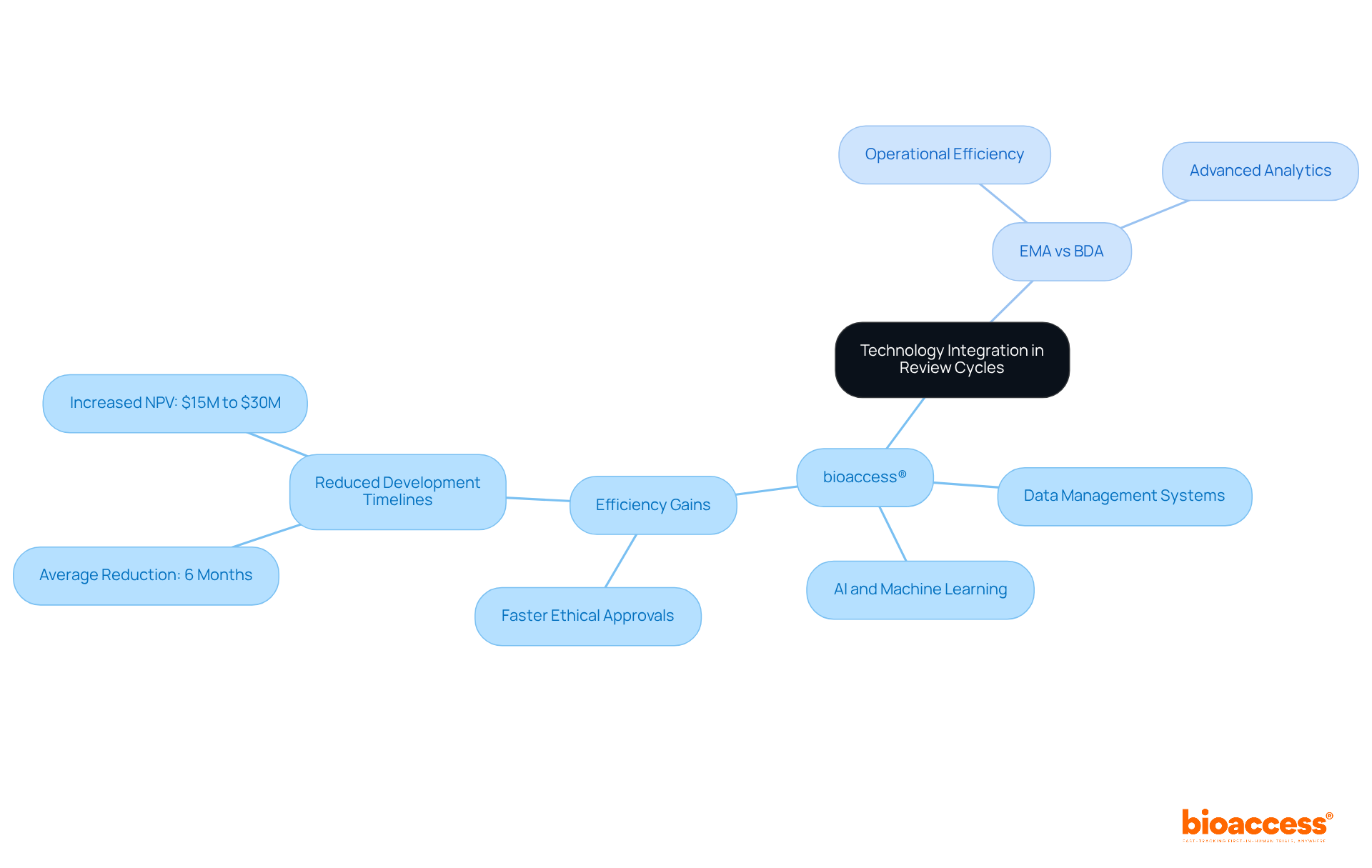
Technology integration is pivotal in enhancing the evaluation cycles by addressing the differences between EMA and BDA review cycles at bioaccess®. By employing sophisticated data management systems, bioaccess® streamlines submissions and monitors progress, achieving ethical approvals in just 4 to 6 weeks - significantly faster than traditional markets. This efficiency is bolstered by the integration of AI and machine learning, which compresses development timelines by an average of six months per asset, adding substantial net present value to sponsors' portfolios.
Meanwhile, the EMA is increasingly adopting digital tools to boost operational efficiency, which illustrates the differences between EMA and BDA review cycles, as BDA leverages advanced analytics to refine data evaluation processes. Together, these technological advancements are essential in reducing evaluation times and enhancing overall outcomes in clinical research.
As the Medtech landscape evolves, the collaboration between these entities underscores the importance of embracing innovation to tackle key challenges in clinical research.
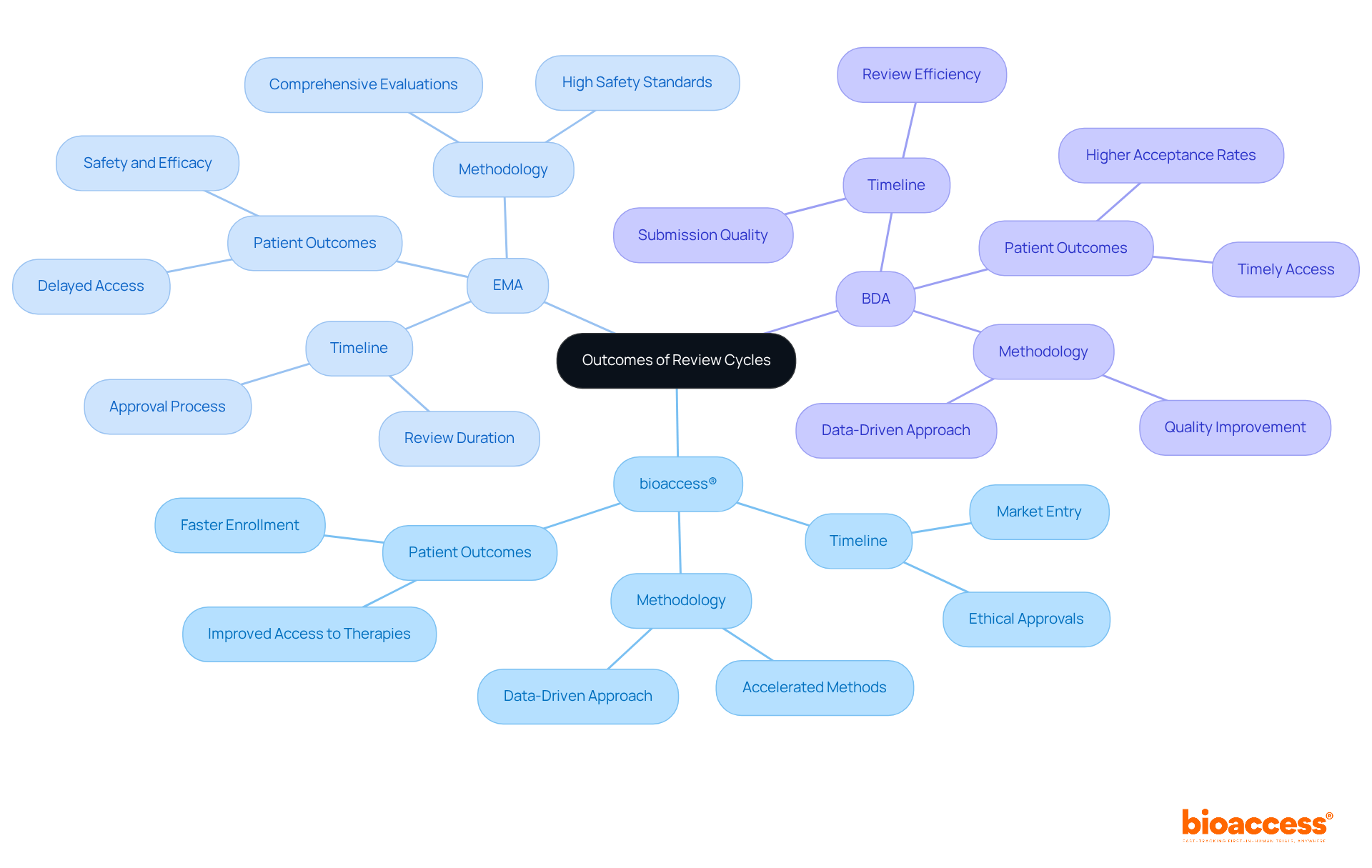
The assessment cycles of bioaccess®, EMA, and BDA demonstrate the differences between EMA and BDA review cycles, which are crucial for clinical research. Bioaccess® employs accelerated methods that facilitate ethical clearances in just 4-6 weeks, enabling market entry for innovative therapies that is 50% faster than traditional markets. This rapid timeline not only enhances patient outcomes but also ensures timely access to critical treatments.
In contrast, the EMA conducts comprehensive evaluations to uphold high safety and efficacy standards, but its review process typically spans 12 to 15 months. This extended duration can delay patient access to new therapies, which is a pressing concern in the Medtech landscape. Meanwhile, BDA adopts a data-driven approach that improves the quality of submissions, potentially leading to better acceptance rates and timelines.
For instance, therapies developed through bioaccess® have demonstrated quicker market entry, underscoring the efficiency of its optimized methods in meeting urgent healthcare demands. The integration of expedited pathways not only accelerates the assessment process but also directly improves patient outcomes, as prompt access to therapies is vital for effective treatment.
In summary, understanding these differences is essential for stakeholders in clinical research. Collaboration among these entities can pave the way for innovative solutions that address key challenges in the industry.
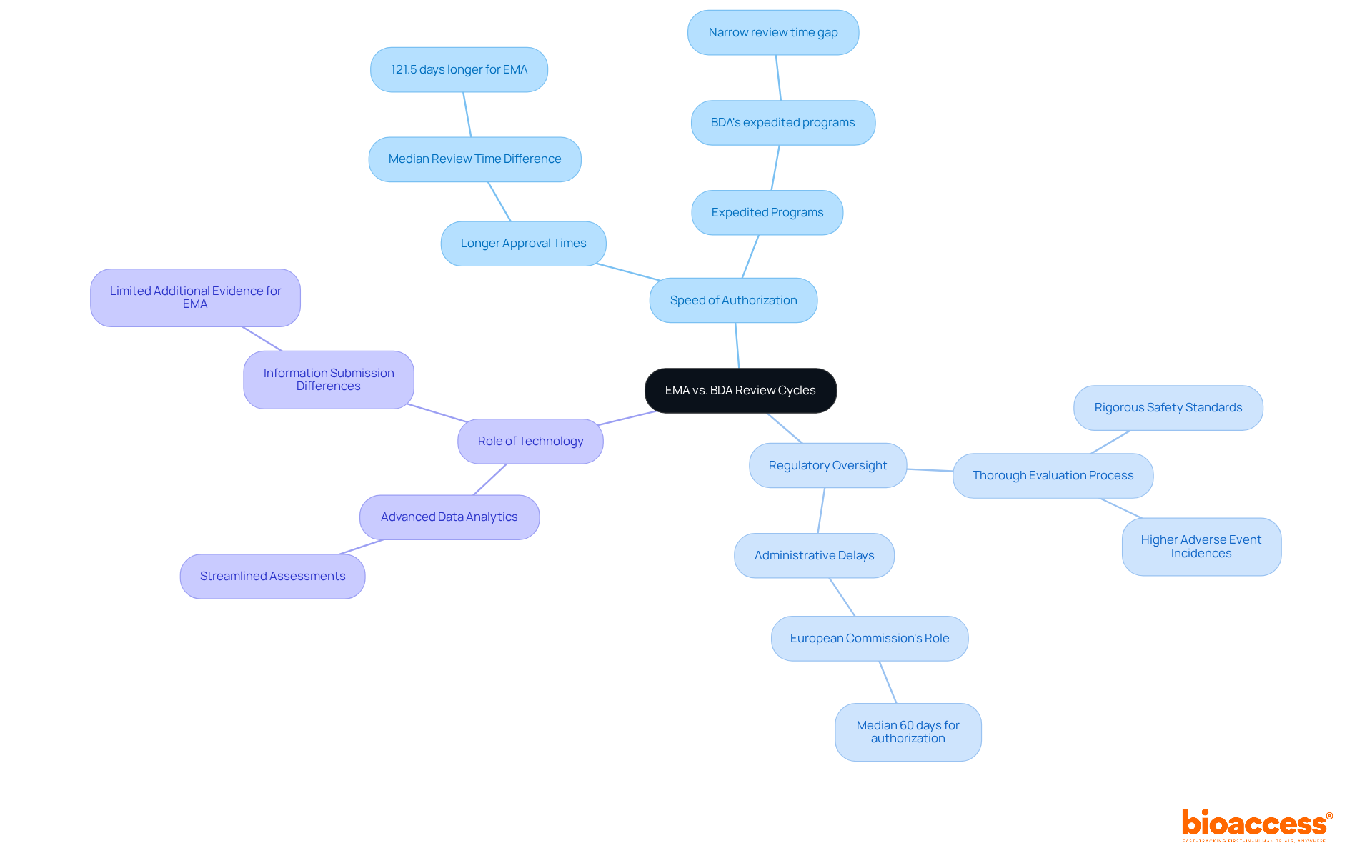
The differences between EMA and BDA review cycles are crucial for anyone involved in clinical research. They primarily revolve around the speed of authorization, levels of regulatory oversight, and the role of technology. The EMA's thorough evaluation process often results in longer approval times, underscoring its commitment to rigorous safety and efficacy standards. In contrast, BDA methodologies utilize advanced data analytics to streamline assessments, allowing for quicker evaluations.
For example, the median review time for drugs approved by the EMA can exceed that of the FDA by approximately 121 days. However, BDA's expedited programs can significantly narrow this gap. When developing clinical research strategies, stakeholders must consider the differences between EMA and BDA review cycles, as the degree of regulatory scrutiny directly impacts trial timelines and overall project outcomes.
Understanding these dynamics is essential for optimizing the development and market entry of new therapeutics. By recognizing the distinct approaches of EMA and BDA, researchers can better navigate the complexities of regulatory processes and enhance their strategic planning.
The exploration of the differences between the review cycles of bioaccess®, the European Medicines Agency (EMA), and Big Data Analytics (BDA) underscores critical variations in speed, efficiency, and ethical considerations that shape clinical research. Bioaccess® stands out as a frontrunner, achieving ethical approvals in an impressive 4-6 weeks, in stark contrast to the EMA's more extended 210-day assessment period. This agility not only accelerates the pathway for innovative therapies but also enhances the competitive landscape for companies operating within Latin America.
Key insights reveal that:
Ultimately, understanding these differences is vital for stakeholders in clinical research. By recognizing how bioaccess®, EMA, and BDA approach review cycles, researchers can better position themselves to optimize development strategies and ensure timely access to critical therapies. Embracing these insights will not only enhance project outcomes but also contribute to advancing healthcare solutions in a rapidly evolving landscape.
What is bioaccess® and how does it impact clinical research review cycles in Latin America?
bioaccess® leverages the regulatory speed of Latin America, particularly Colombia, to significantly shorten clinical research review cycles. It achieves ethical consent in 4-6 weeks, much faster than the typical several months in conventional markets, thus accelerating the path to market for Medtech, Biopharma, and Radiopharma innovators.
How does bioaccess® improve patient enrollment for clinical trials?
bioaccess® can secure patient enrollment 50% faster than traditional sites, which helps firms focused on innovative treatments navigate the validation process more quickly.
What advantages does Colombia's healthcare system offer for clinical research?
Colombia's healthcare system, recognized as one of the best in Latin America, supports robust patient recruitment with a population of over 50 million and universal healthcare coverage. Additionally, the region offers substantial R&D tax incentives, including a 100% tax deduction for investments in science, technology, and innovation projects.
What is the projected growth of the Latin American clinical trials market?
The Latin American clinical trials market is projected to grow at a compound annual growth rate of 7.9% from 2025 to 2033, driven by the region's regulatory efficiency.
What is the role of the European Medicines Agency (EMA) in medication evaluation?
The EMA ensures the safety and effectiveness of medications through a structured evaluation cycle for standard applications, typically lasting 210 days, which includes initial assessments, detailed evaluations, and designated clock stops for applicant inquiries.
What are the key stages in the EMA's review cycle?
The key stages in the EMA's review cycle include an initial assessment, detailed evaluation, and two significant clock stops: the first at Day 120 for 3-6 months for applicants to respond to questions, and the second at Day 180 for 1-2 months to resolve outstanding issues.
How has the submission timeliness of marketing authorization applications (MAAs) been in recent years?
In 2023, only 35% of marketing authorization applications (MAAs) were submitted on time, highlighting the complexities involved in the EMA's review procedure.
What is the impact of Big Data Analytics (BDA) on regulatory assessment cycles?
BDA is revolutionizing regulatory assessment cycles by identifying trends, streamlining workflows, and enhancing decision-making efficiency, resulting in authorization times that can be up to 50% faster than traditional methods.
How does bioaccess® utilize Big Data Analytics in its processes?
bioaccess® employs a 'Patient Velocity' approach, achieving 50% faster patient enrollment and saving $25K per patient by providing FDA-ready data, which reduces rework and delays.
What are the benefits of partnerships in clinical research, as demonstrated by bioaccess®?
Partnerships, such as that of bioaccess with GlobalCare Clinical Trials in Colombia, have led to over a 50% reduction in recruitment time and a retention rate exceeding 95%, showcasing the effectiveness of collaborative methodologies in clinical research.