


Navigating the pediatric clinical trial landscape demands a thorough understanding of the regulatory frameworks governing research involving children. At the forefront of this process in Croatia is Halmed, the agency tasked with ensuring that trials are conducted ethically and safely. This guide serves as a comprehensive roadmap for sponsors seeking approval for pediatric clinical trials, detailing essential documentation, submission steps, and common challenges encountered along the way.
What strategies can researchers employ to streamline their application process and enhance their chances of timely approval from Halmed?
The Croatian Agency for Medicinal Products and Medical Devices plays a pivotal role in overseeing research studies, particularly those involving pediatric populations. This agency is responsible for ensuring that all medical studies comply with national and European regulations, thereby safeguarding the rights and welfare of child participants. Understanding the regulatory body's functions, such as:
is essential for any sponsor aiming to achieve pediatric clinical trial approval by halmed for conducting studies in Croatia.
Moreover, the agency collaborates with ethics committees to ensure that studies are designed and executed ethically, especially when dealing with vulnerable groups like children. This collaboration not only reinforces the integrity of the research but also builds trust among stakeholders. As sponsors navigate the complexities of pediatric research, recognizing the agency's role in obtaining pediatric clinical trial approval by halmed can significantly enhance the quality and compliance of their studies.

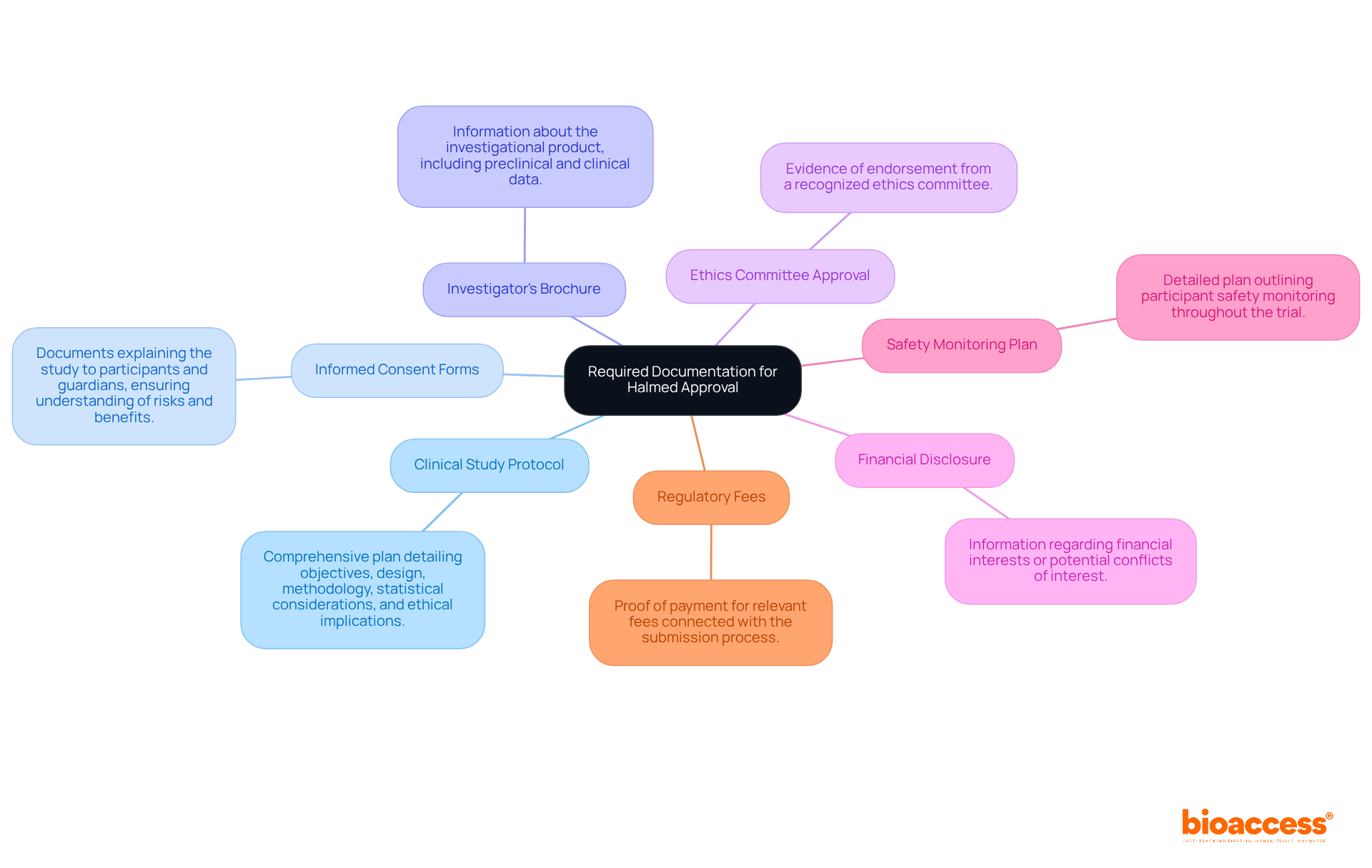
To successfully submit an application to Halmed for pediatric clinical trials, it is essential to compile the following documentation:
To prevent delays in the review process, ensure that all documents are complete, accurate, and formatted according to the established guidelines for pediatric clinical trial approval by Halmed.
To successfully submit your pediatric clinical trial application to Halmed, follow these essential steps:
Prepare Your Application: Ensure all documents are complete and comply with the submission guidelines. This includes preparing both electronic and paper copies as required. Remember to format your submission according to the Common Technical Document (CTD) guidelines and ensure that all documentation is submitted in Croatian or English.
Online Submission: Access the Halmed online submission portal. If you do not have an account, create one. Complete the electronic registration form, ensuring all fields are filled out accurately.
Upload Documentation: Attach all necessary documents to your submission. Verify that each document is correctly labeled and formatted (PDF, DOCX, etc.).
Payment of Fees: Confirm that you have paid the administrative fee of 70.00 kn related to your submission. Retain a receipt or confirmation of payment as proof, as this is a critical requirement for your submission.
Submit the Form: Conduct a final review of your submission for completeness and accuracy before sending it through the portal. Be aware that HALMED may inform you of any errors in your submission, so double-checking is essential.
Confirmation: After submission, you will receive a confirmation email from the organization. Keep this email for your records, as it serves as proof of submission.
Following these steps will promote a smooth submission process and improve the chances of approval. Additionally, consider reviewing success rates of submissions made to Halmed to better understand the approval landscape.

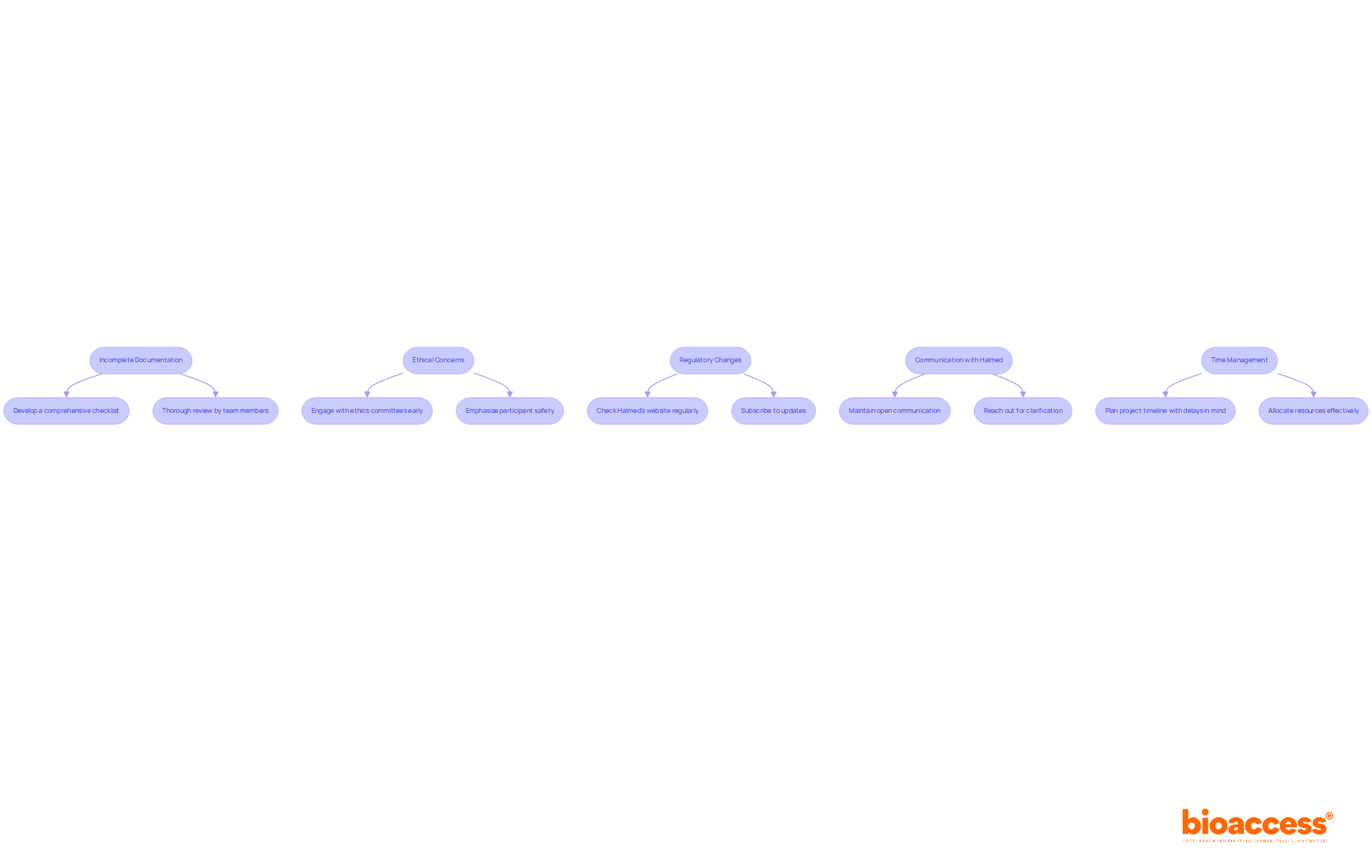
Navigating the pediatric clinical trial approval by Halmed is crucial for successful outcomes in clinical research. Understanding the challenges involved can significantly enhance your chances of timely approval. Here are some common issues and effective strategies to address them:
Incomplete Documentation: Incomplete or improperly formatted documentation is a leading cause of application delays. To mitigate this risk, develop a comprehensive checklist aligned with Halmed's requirements. Ensure that all documents undergo thorough review by multiple team members before submission to enhance accuracy and completeness.
Ethical Concerns: Pediatric studies are subject to rigorous ethical scrutiny. Engage with ethics committees early in the process to proactively address potential concerns. Design your trial with a strong emphasis on participant safety and welfare, ensuring that ethical considerations are integrated into every aspect of the study.
Regulatory Changes: The regulatory landscape is dynamic, and staying informed about changes that may impact your application is crucial. Consistently check the organization's website and subscribe to updates to stay compliant with the latest guidelines and requirements.
Communication with Halmed: Establishing and maintaining open lines of communication with Halmed is essential throughout the approval process. Should you encounter challenges or have questions, do not hesitate to reach out for clarification or guidance. This proactive communication can help resolve issues more efficiently.
Time Management: The approval process can be lengthy, often leading to delays. It is vital to plan your project timeline with these potential delays in mind. By allowing for extra time in your schedule, you can better manage expectations and allocate resources effectively.
By anticipating these challenges and preparing accordingly, you can significantly enhance your chances of a successful application and obtain pediatric clinical trial approval by Halmed in a timely manner.
Achieving pediatric clinical trial approval by Halmed is not just a complex process; it’s an essential one that safeguards the safety and welfare of child participants in medical research. Understanding the regulatory framework and Halmed's role in overseeing these trials is crucial for sponsors who aim to navigate the approval landscape effectively. By recognizing the importance of compliance with national and European regulations, sponsors can enhance the integrity of their studies and build trust with stakeholders.
This article outlines the key steps involved in securing approval, including:
Essential documents such as the Clinical Study Protocol, Informed Consent Forms, and Ethics Committee Approval are highlighted as critical components that must be meticulously compiled and submitted. Moreover, addressing ethical concerns, maintaining open communication with Halmed, and managing project timelines are vital aspects that cannot be overstated.
In a field where the stakes are high, the significance of thorough preparation and proactive engagement with regulatory bodies like Halmed is paramount. By following the outlined steps and anticipating potential challenges, sponsors can not only improve their chances of timely approval but also contribute to the advancement of pediatric research. Embracing these guidelines will ultimately lead to more effective and ethically sound clinical trials that prioritize the health and safety of young participants.
What is Halmed's role in pediatric clinical trials?
Halmed, the Croatian Agency for Medicinal Products and Medical Devices, oversees research studies involving pediatric populations, ensuring compliance with national and European regulations to protect the rights and welfare of child participants.
What are the key responsibilities of Halmed in pediatric clinical trials?
Halmed is responsible for evaluating research protocols, considering ethical aspects, and overseeing safety to ensure that studies are conducted properly.
Why is it important for sponsors to understand Halmed's functions?
Understanding Halmed's functions is essential for sponsors seeking pediatric clinical trial approval, as it helps them navigate the regulatory landscape and enhances the quality and compliance of their studies.
How does Halmed collaborate with ethics committees?
Halmed collaborates with ethics committees to ensure that studies involving children are designed and executed ethically, reinforcing research integrity and building trust among stakeholders.
What impact does Halmed's oversight have on pediatric research?
Halmed's oversight enhances the ethical conduct of pediatric research and ensures that the rights and welfare of vulnerable child participants are prioritized throughout the study process.