


Understanding the complexities of Serbian Good Clinical Practice (GCP) is crucial for improving the quality of clinical research in the region. This article explores effective strategies for training local investigators, emphasizing the necessity of adhering to both local regulations and international standards. As the landscape of clinical trials continues to evolve, organizations must consider: how can they ensure their training programs not only fulfill current requirements but also equip researchers for future challenges?
By addressing these questions, we can better navigate the Medtech landscape and the role of bioaccess in overcoming key challenges. Collaboration and proactive training are essential for fostering a robust clinical research environment that meets both present and future demands.
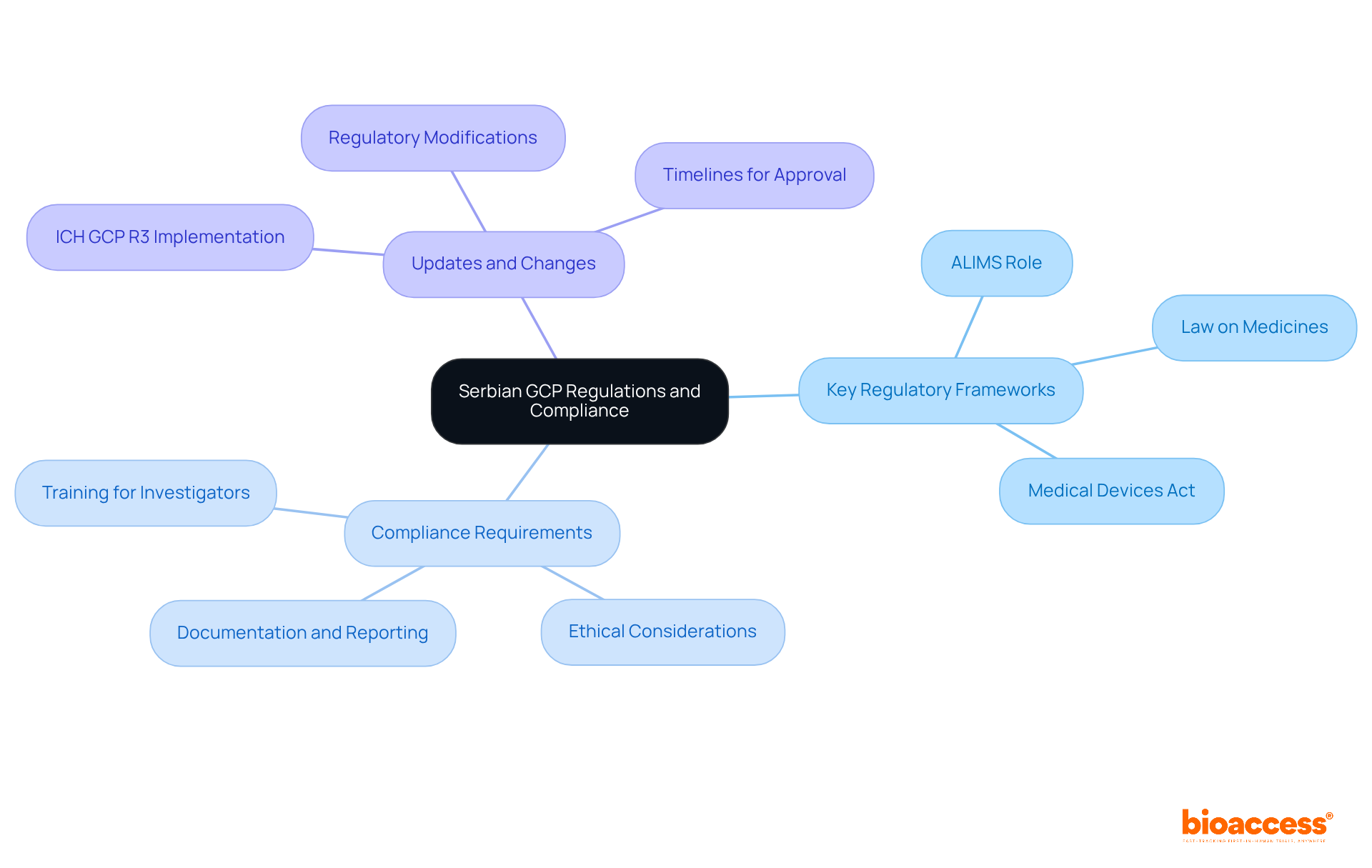
A thorough understanding of Serbian Good Clinical Practice (GCP) regulations is essential for training local investigators under Serbian GCP. Serbian GCP aligns with the International Council for Harmonisation (ICH) guidelines, particularly the ICH E6(R3) standards, which emphasize the ethical and scientific quality of research trials. Researchers must be well-versed in local regulations governing medical studies, including the Law on Medicines and the Medical Devices Act, which delineate the responsibilities of both researchers and sponsors.
Training programs should encompass detailed modules on the following:
Grounding education in these regulations allows researchers to guarantee that their studies are compliant and ethically sound, ultimately protecting participant welfare and enhancing the credibility of studies conducted in Serbia. Recent trends indicate a significant reduction in regulatory timelines for trials, fostering optimism among sponsors and stakeholders. This further emphasizes the importance of thorough preparation and adherence to GCP standards.
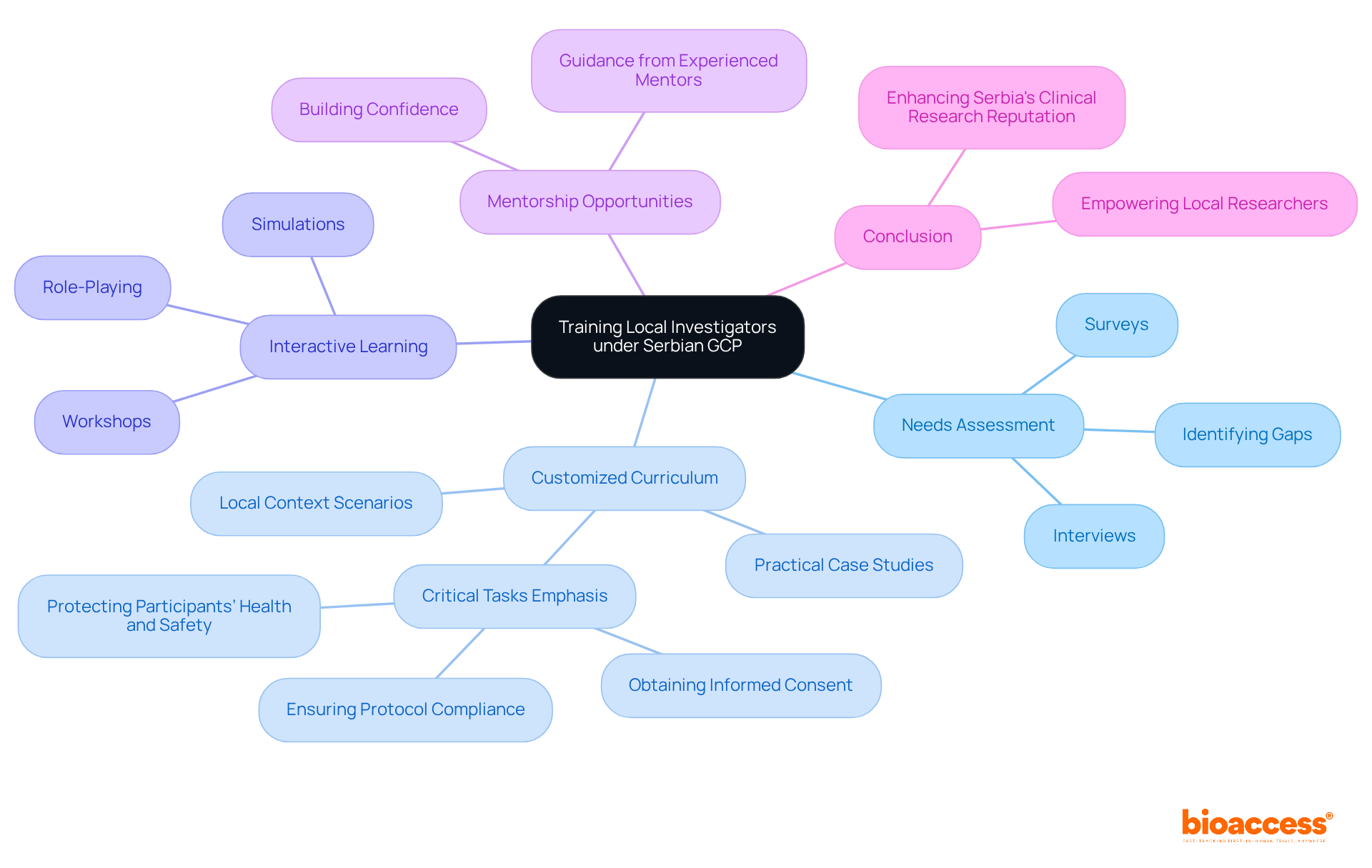
Training local investigators under Serbian GCP is crucial for developing effective educational programs for local professionals and enhancing clinical research in Serbia. This requires a customized approach that considers their unique backgrounds, experiences, and the specific challenges they face in conducting studies. By focusing on training local investigators under Serbian GCP, organizations can empower local researchers to conduct high-quality studies that meet both local and global standards, thereby elevating Serbia's reputation as a center for clinical trials.
Needs Assessment: Conducting surveys or interviews with local investigators is essential for the purpose of training local investigators under Serbian GCP to identify gaps in knowledge and skills related to Good Clinical Practice (GCP) and clinical trial management. A recent survey highlighted a significant deficiency in formal education regarding ethics in research among Serbian researchers. This gap underscores the urgent need for targeted instruction, particularly in training local investigators under Serbian GCP, as ethical conduct is paramount in research integrity.
Customized Curriculum: It is vital to develop a curriculum that directly addresses these identified needs. This curriculum should incorporate practical case studies and examples that resonate with the Serbian context, including scenarios involving local patient groups and regulatory challenges, while training local investigators under Serbian GCP. Emphasizing the top three critical tasks for trial quality-obtaining informed consent, ensuring protocol compliance, and protecting participants’ health and safety-through training local investigators under Serbian GCP will ensure that the instruction is both relevant and beneficial.
Interactive Learning: Engaging researchers through interactive educational methods such as workshops, role-playing, and simulations is key to training local investigators under Serbian GCP. This hands-on approach not only fosters skill development in a safe environment but also builds confidence and proficiency. Addressing the challenges of high practice frequency and repetitive content in GCP preparation can significantly improve the effectiveness of these educational methods.
Mentorship Opportunities: Pairing local researchers with experienced mentors can provide invaluable guidance throughout their development process. This mentorship, which focuses on training local investigators under Serbian GCP, fosters a deeper understanding of best practices and boosts researchers' confidence, ultimately leading to improved trial quality.
In conclusion, by concentrating on customized training, organizations can focus on training local investigators under Serbian GCP to conduct high-quality studies that align with both local and global standards. This strategic focus not only enhances the capabilities of researchers but also strengthens Serbia's position in the global clinical research landscape.
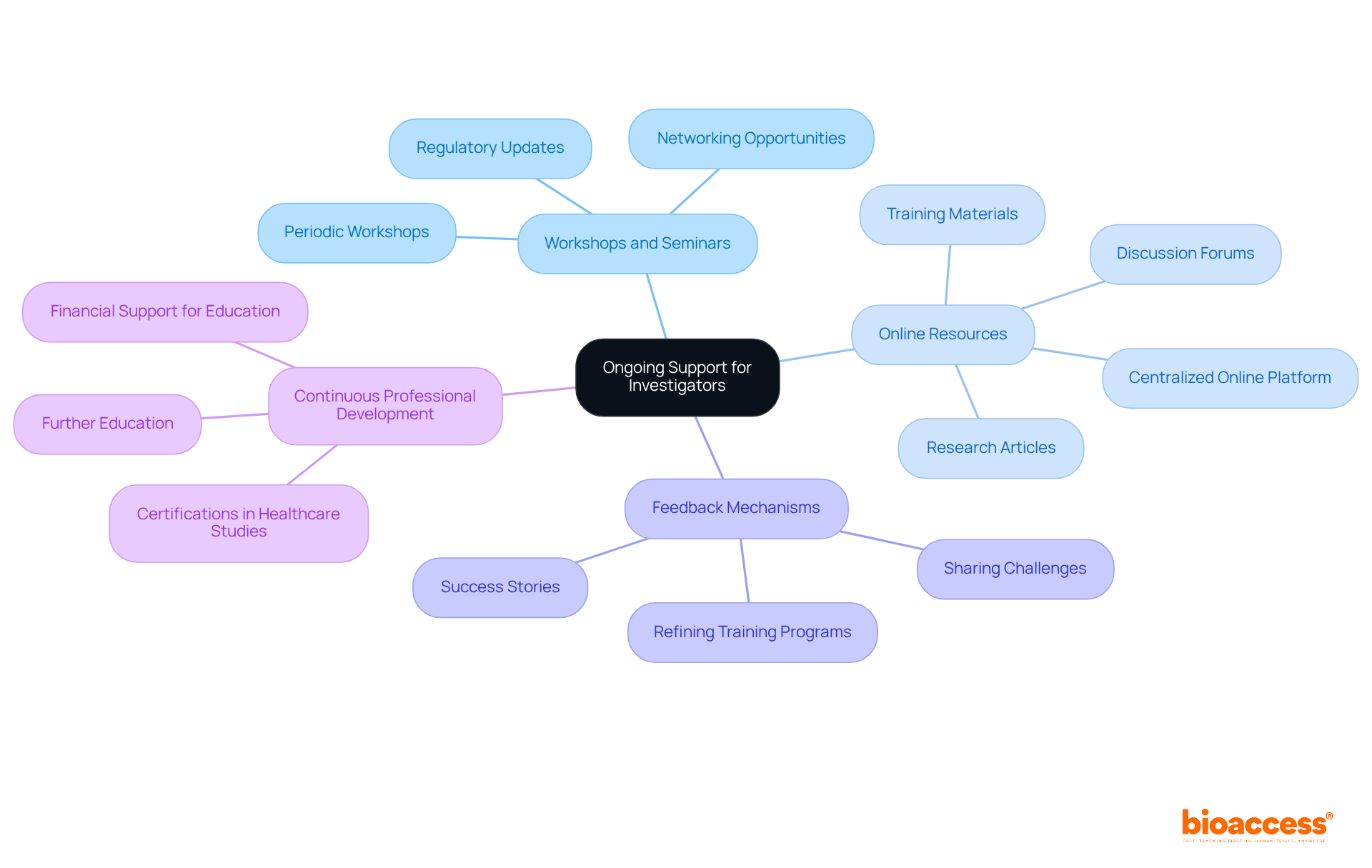
To ensure local examiners are equipped to navigate the evolving landscape of health studies, training local investigators under Serbian GCP is essential for providing ongoing support and resources. This can be accomplished through:
Regular Workshops and Seminars: Periodic workshops should be organized to address new developments in Good Clinical Practice (GCP), regulatory changes, and emerging trends in clinical research. These sessions not only enhance knowledge but also serve as valuable networking opportunities for researchers to exchange experiences and best practices.
Access to Online Resources: Establish a centralized online platform where researchers can easily access training materials, regulatory updates, and relevant research articles. This resource hub can also feature forums for discussion and collaboration among researchers, fostering a sense of community.
Feedback Mechanisms: Implement systems that allow researchers to share their challenges and successes. This feedback can be instrumental in refining training programs and support initiatives, ensuring they remain relevant and effective.
Continuous Professional Development: Encourage researchers to pursue further education and certifications in healthcare studies and GCP. Offering financial support or incentives for these endeavors can significantly enhance their skills and knowledge.
By building a robust support system, organizations can empower local researchers through training local investigators under Serbian GCP to effectively manage the complexities of medical studies, ultimately leading to improved study outcomes and participant safety.
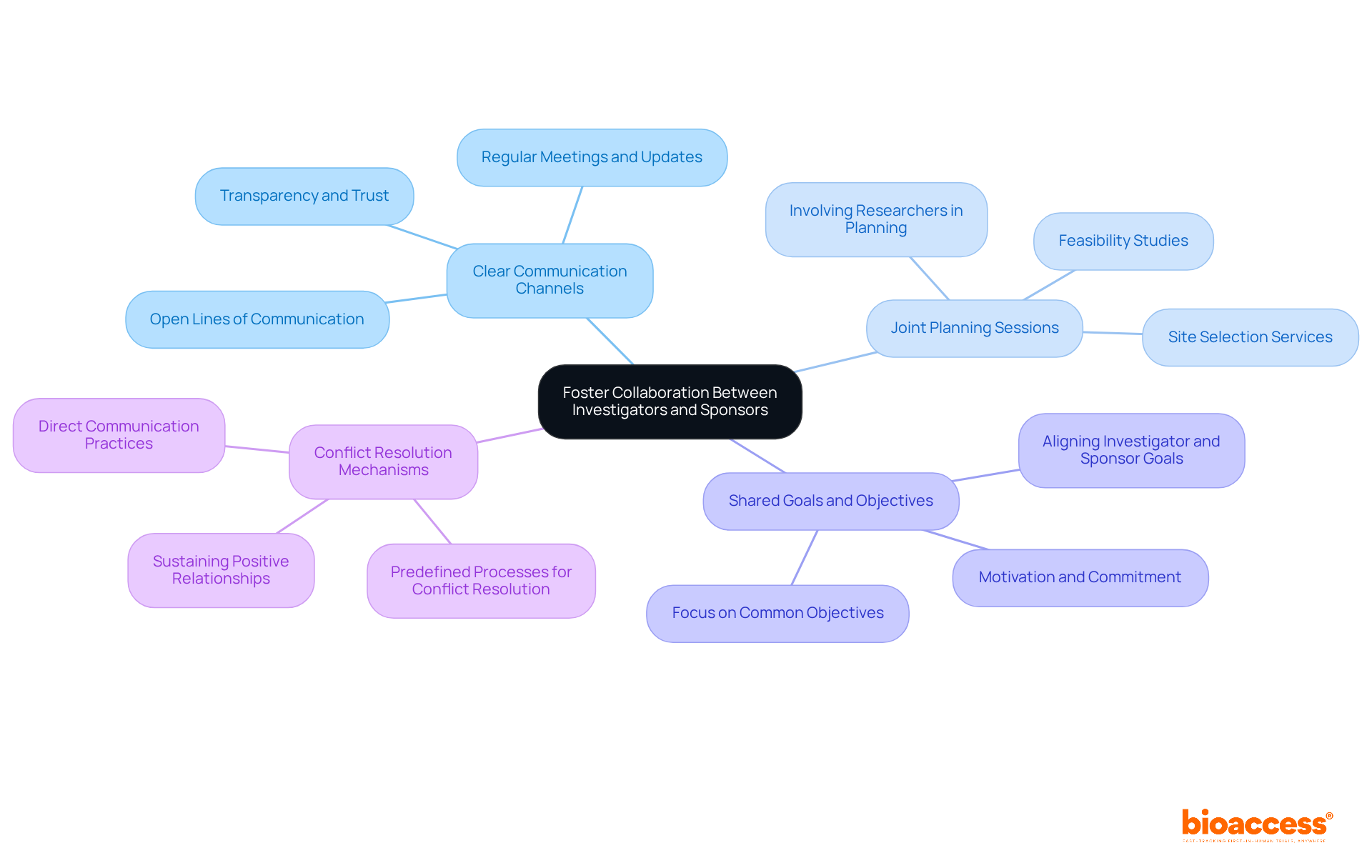
Effective cooperation between researchers and sponsors is crucial for the success of clinical trials. To enhance this collaboration, organizations should implement the following strategies:
Clear Communication Channels: Establishing open lines of communication is vital. Regular meetings and updates ensure that all parties are aligned on study progress, challenges, and expectations, fostering transparency and trust. Bioaccess emphasizes this through its trial management services, ensuring compliance with country requirements and facilitating smooth project execution.
Joint Planning Sessions: Involving researchers in the planning stages allows their insights and expertise to shape study design and protocols. This inclusion leads to more practical and feasible research plans, ultimately enhancing the study's operational efficiency. Bioaccess supports this approach by providing feasibility studies and site selection services that align with researcher input.
Shared Goals and Objectives: Aligning the goals of both investigators and sponsors is essential. When all parties work towards common objectives, it enhances motivation and commitment, driving the study towards success. Bioaccess's project management services are designed to facilitate this alignment, ensuring that all stakeholders are focused on shared objectives.
Conflict Resolution Mechanisms: Developing predefined processes for addressing conflicts or misunderstandings can facilitate quick resolutions. This proactive approach helps sustain a positive working relationship, crucial for managing the complexities of research trials. Bioaccess incorporates direct communication practices into its management approach, fostering a collaborative environment.
By fostering collaboration through these strategies, organizations can create a more cohesive and effective research environment, particularly through training local investigators under Serbian GCP, leading to improved outcomes for clinical trials conducted in Serbia.
Training local investigators under Serbian Good Clinical Practice (GCP) is crucial for maintaining the integrity and quality of clinical research in Serbia. By prioritizing comprehensive education that aligns with local regulations and international standards, organizations can empower researchers to conduct studies that are both ethically sound and scientifically robust.
This article outlines several key strategies for effective training programs. These include:
Furthermore, emphasizing the importance of mentorship and collaboration between researchers and sponsors enhances the capacity for successful clinical trials, ensuring that all stakeholders work towards shared objectives.
Ultimately, investing in the training and support of local investigators not only elevates the quality of research conducted in Serbia but also strengthens the country’s position in the global clinical research landscape. By implementing these best practices, stakeholders can foster a culture of excellence in clinical trials, leading to improved outcomes for participants and the advancement of medical science.
Why is understanding Serbian GCP regulations important for researchers?
Understanding Serbian Good Clinical Practice (GCP) regulations is essential for training local investigators and ensuring that research trials are conducted ethically and scientifically in accordance with local laws.
How do Serbian GCP regulations align with international standards?
Serbian GCP regulations align with the International Council for Harmonisation (ICH) guidelines, particularly the ICH E6(R3) standards, which emphasize the ethical and scientific quality of research trials.
What local regulations must researchers be familiar with when conducting studies in Serbia?
Researchers must be well-versed in the Law on Medicines and the Medical Devices Act, which outline the responsibilities of both researchers and sponsors in medical studies.
What key topics should training programs cover regarding Serbian GCP?
Training programs should cover key regulatory frameworks, compliance requirements, and regular updates on any regulatory changes, particularly related to the implementation of ICH GCP R3 in July 2025.
What is the role of the Agency for Medicines and Medical Devices (ALIMS) in Serbian clinical trials?
The Agency for Medicines and Medical Devices (ALIMS) is a regulatory body responsible for overseeing clinical trials in Serbia, ensuring compliance with GCP standards.
What are some compliance requirements researchers must adhere to during studies?
Researchers must understand necessary documentation, reporting obligations, and ethical considerations that must be followed throughout the study.
What recent trends have been observed regarding regulatory timelines for trials in Serbia?
Recent trends indicate a significant reduction in regulatory timelines for trials, which has fostered optimism among sponsors and stakeholders.
How does grounding education in Serbian GCP regulations benefit research studies?
Grounding education in these regulations helps ensure that studies are compliant and ethically sound, protecting participant welfare and enhancing the credibility of studies conducted in Serbia.