


Navigating the complexities of clinical trials in Serbia presents both challenges and opportunities, particularly in the realm of adaptive design. Researchers aiming to optimize their trial logistics can gain valuable insights into:
But how can one effectively master these logistics to ensure a successful outcome in an ever-evolving regulatory landscape? This article explores four key practices that empower researchers to enhance their adaptive trial processes in Serbia, ultimately leading to more efficient and effective studies.
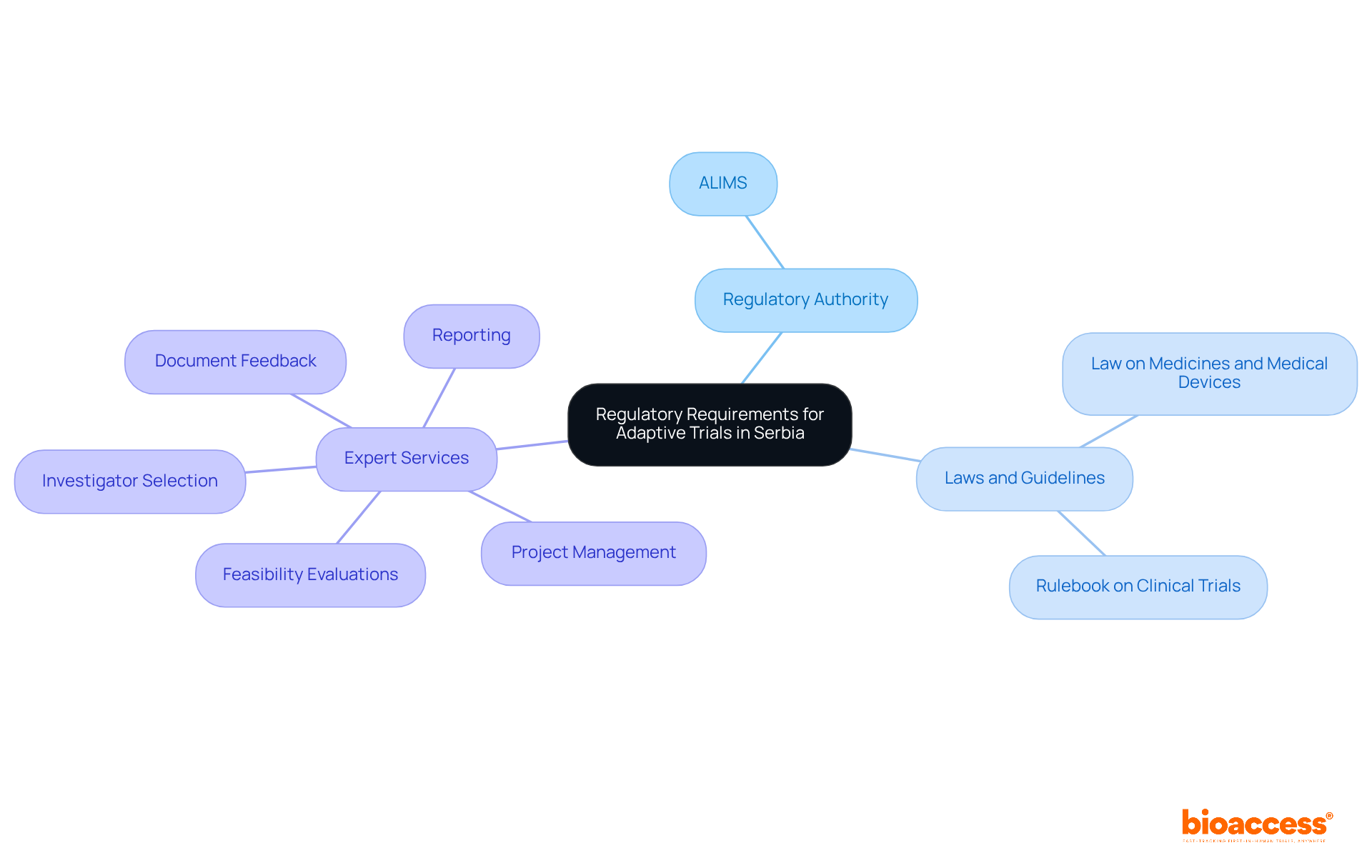
Navigating the regulatory landscape for adaptive design trial logistics in Serbia requires a comprehensive understanding of local laws and guidelines. The Medicines and Medical Devices Agency of Serbia (ALIMS) serves as the primary authority overseeing clinical research approvals. Familiarity with their requirements is essential, particularly the Law on Medicines and Medical Devices and the Rulebook on Clinical Trials, which outline the necessary documentation, ethical considerations, and approval timelines. The adaptive design trial logistics in Serbia must adhere to Good Clinical Practice (GCP) principles, ensuring that any modifications to the study design receive prior approval from regulatory authorities.
Engaging local regulatory experts, such as those at bioaccess, can significantly streamline the navigation of these requirements. Bioaccess offers extensive services, including:
This comprehensive support accelerates the approval process and enhances the likelihood of successful outcomes. Their expertise in connecting innovative Medtech, Biopharma, and Radiopharma startups with leading clinical research facilities ensures that studies progress to the next phase more swiftly, providing a competitive edge in the market.
In summary, collaboration with local experts like bioaccess is crucial for navigating the complexities of clinical research in Serbia. By leveraging their knowledge and resources, researchers can effectively address regulatory challenges and enhance their chances of success.
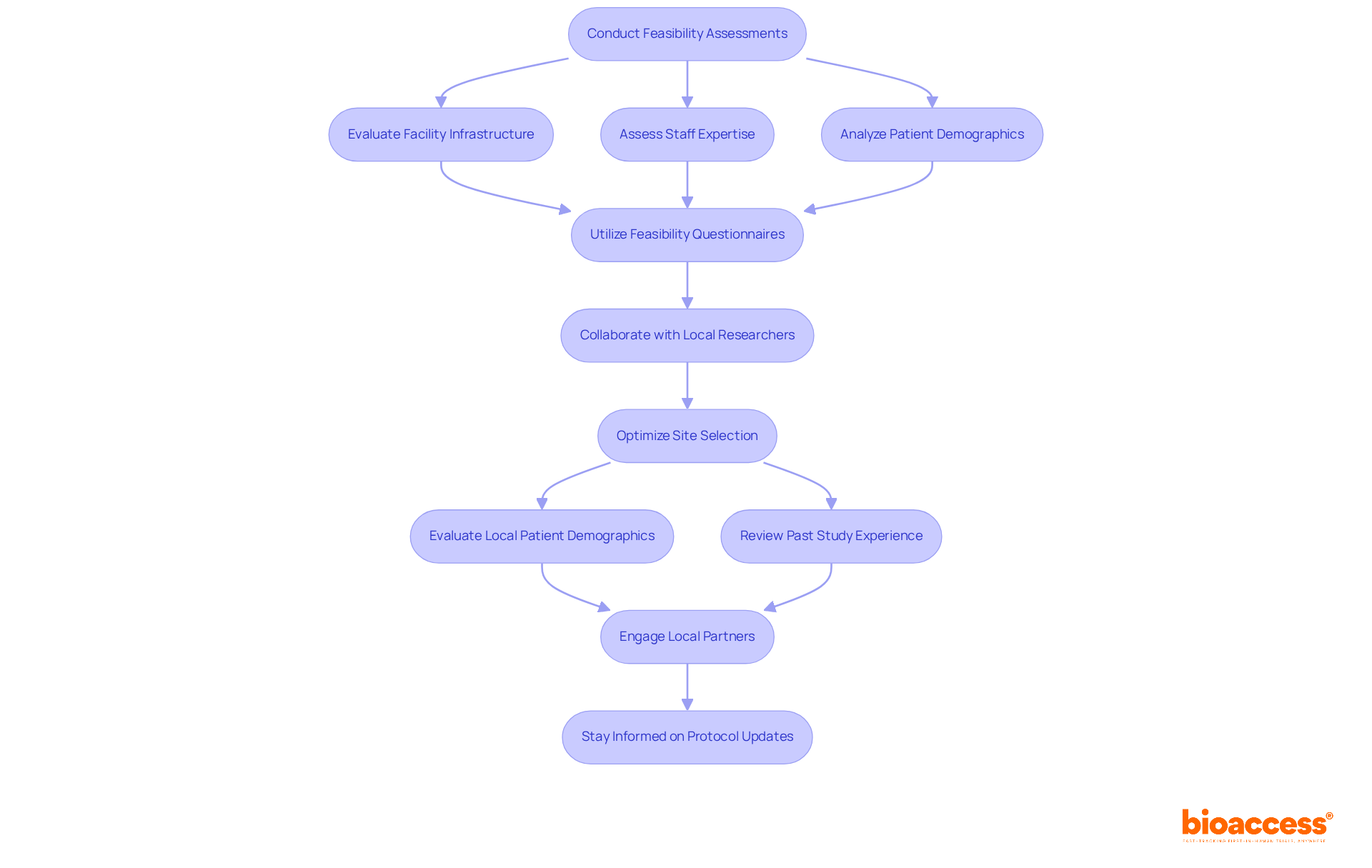
Feasibility evaluations are essential in determining the suitability of clinical research locations. This thorough assessment encompasses the facility's infrastructure, staff expertise, and patient demographics. In Serbia, where clinical trial sites are primarily located in major urban centers like Belgrade, it’s vital to evaluate adaptive design trial logistics in Serbia as well as analyze the historical performance of these locations in previous trials. By utilizing tools such as feasibility questionnaires and collaborating with local researchers, we can gain critical insights into the readiness of these sites.
bioaccess offers a comprehensive suite of services that includes feasibility assessments and the selection of research locations and principal investigators (PIs). This ensures compliance with country requirements through meticulous review and feedback on study documents. Moreover, optimizing site selection involves evaluating local patient demographics and the site’s past study experience, which can significantly enhance recruitment efforts and ensure that enrollment goals are met effectively. Engaging local partners and understanding cultural nuances can foster trust and improve participant retention, ultimately driving the success of adaptive design trial logistics in Serbia.
Looking ahead, with updates to Serbia's research protocols anticipated in 2025, it is imperative to stay informed about these changes. Navigating the evolving landscape of clinical studies will require adaptability and foresight, making collaboration more important than ever.
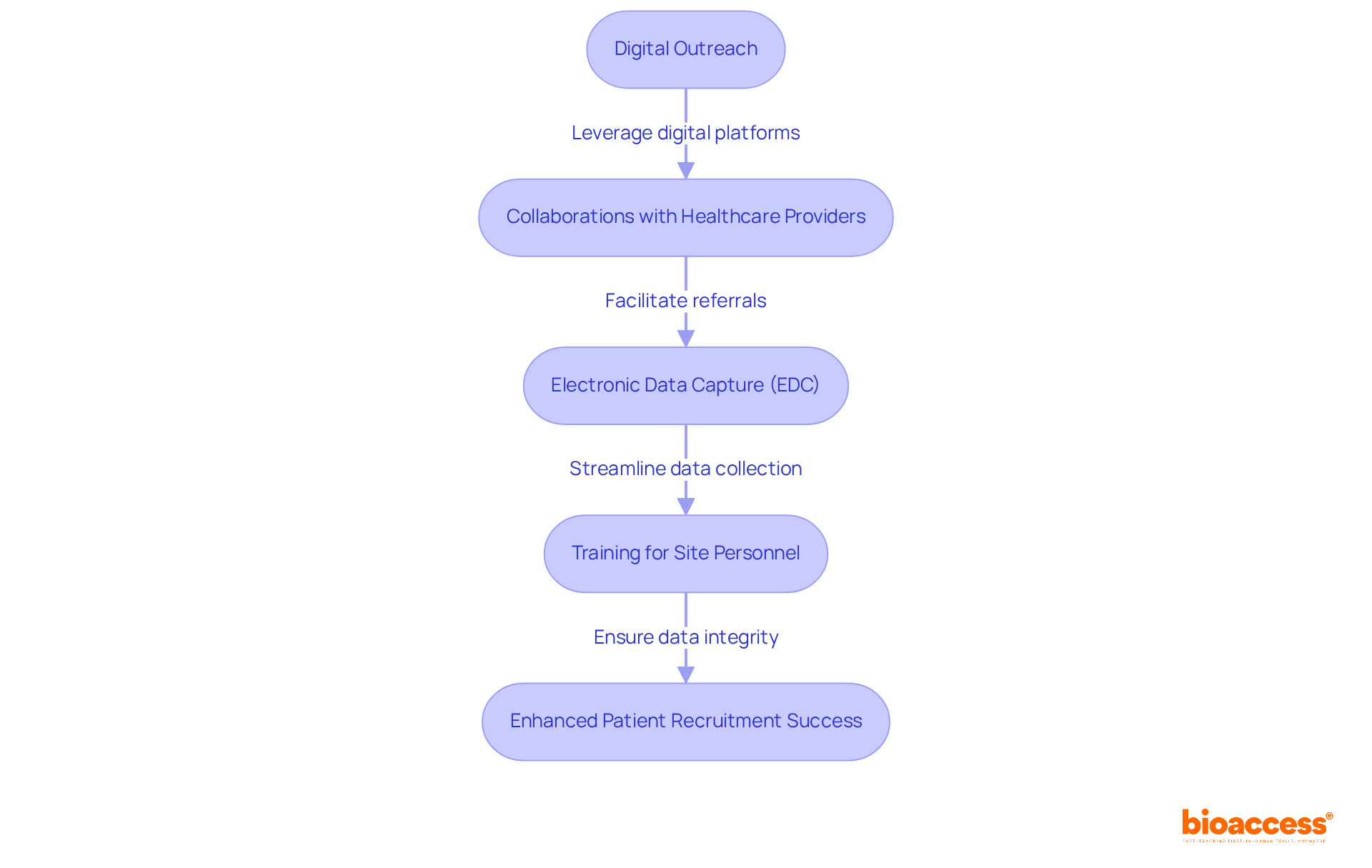
Efficient participant recruitment strategies are crucial in the adaptive design trial logistics in Serbia, where timely enrollment significantly impacts success. In Serbia, leveraging digital platforms and social media can greatly enhance outreach to potential participants involved in adaptive design trial logistics in Serbia. For example, investigators screened 18 individuals within just four weeks, showcasing the effectiveness of these methods.
Forming collaborations with local healthcare providers is essential for facilitating patient referrals and raising awareness about the study. Additionally, effective information management plays a vital role; implementing electronic data capture (EDC) systems streamlines information collection and ensures immediate access to study details. This approach not only improves the accuracy of information but also supports compliance with regulatory requirements, as the review process for research studies in Serbia typically takes around 60 days.
Consistent training for site personnel on data management protocols can mitigate risks associated with data integrity, ensuring that studies maintain high standards of quality and efficiency. By prioritizing these strategies, researchers can enhance patient recruitment and contribute to the overall success of clinical studies, particularly in the context of adaptive design trial logistics in Serbia.
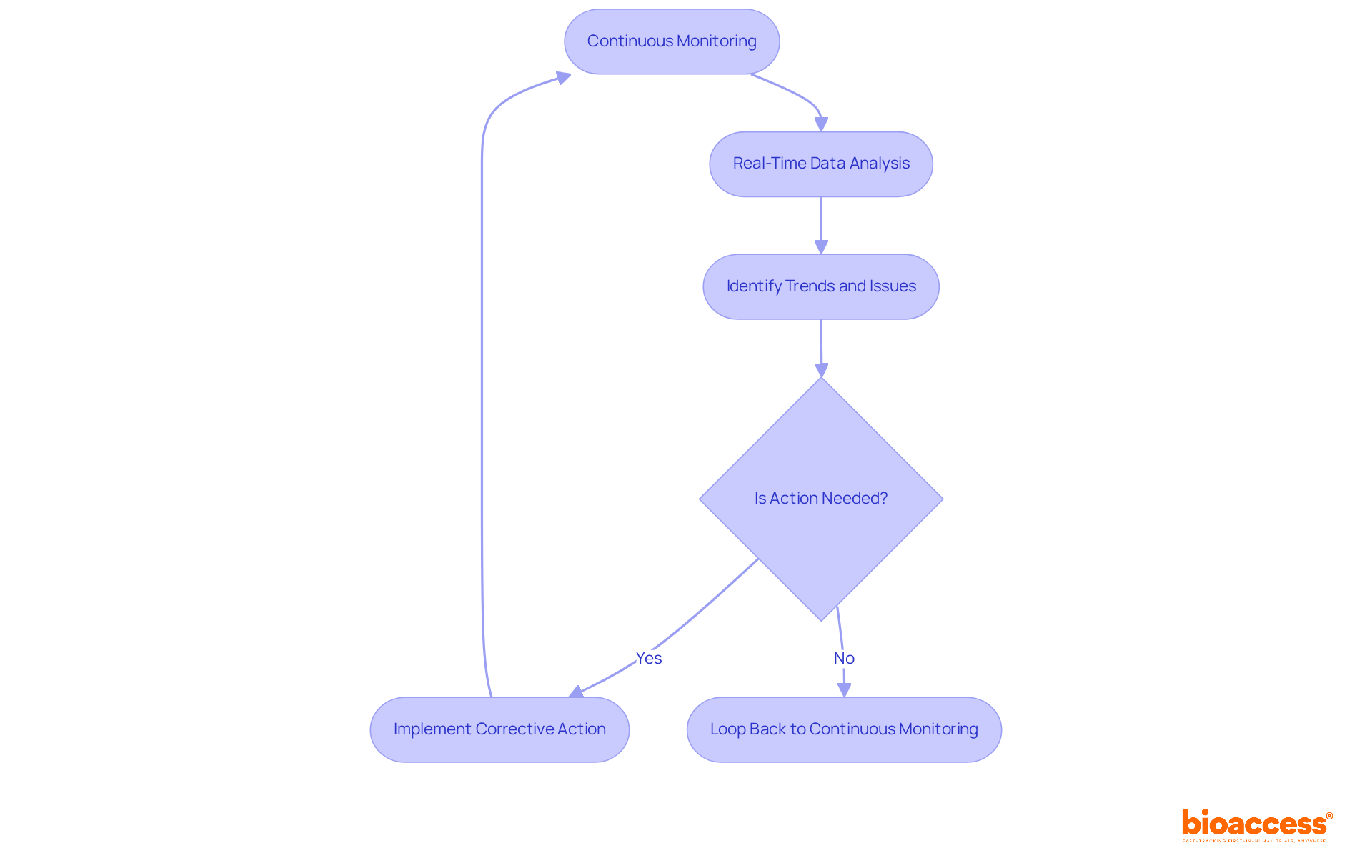
Continuous monitoring is crucial in adaptive design trial logistics in Serbia, as it allows for swift modifications based on interim outcomes. By implementing a centralized monitoring system, trial managers can perform real-time data analysis, which helps identify trends and potential issues early on. For example, in a diabetes study, centralized monitoring uncovered a site with an unusual lack of variability in vital signs, leading to immediate investigation and corrective action. This proactive approach not only enhances patient safety but also ensures compliance with regulatory standards, as highlighted by the 2017 ICH E6 (R2) addendum.
Flexibility in experimentation is equally vital; the adaptive design trial logistics in Serbia allow for adjustments to the protocol based on gathered information, resulting in more effective resource utilization and improved outcomes. Establishing clear communication channels among all stakeholders, including investigators and regulatory bodies, is essential for facilitating these adaptations. As trial managers have noted, the ability to adapt protocols in response to real-time data significantly boosts overall trial effectiveness.
With bioaccess®'s capabilities, you can enroll treatment-naive cardiology or neurology cohorts 50% faster than Western sites, achieving $25K savings per patient with FDA-ready data-no rework, no delays. This not only streamlines the process but also positions your study for success in a competitive landscape.
Mastering adaptive design trial logistics in Serbia is crucial for researchers who want to navigate the complexities of clinical trials effectively. By understanding the regulatory framework, conducting thorough feasibility assessments, implementing effective patient recruitment strategies, and establishing continuous monitoring, researchers can significantly enhance their chances of achieving successful outcomes.
This article shared key insights on:
The emphasis on continuous monitoring and flexibility in trial execution highlights how real-time data analysis can lead to timely interventions and improved trial effectiveness. Collaborating with local experts, such as bioaccess, streamlines processes and ensures compliance with evolving regulations.
In summary, the landscape of clinical trials in Serbia presents both challenges and opportunities. By adopting these best practices, researchers can enhance their operational efficiency and contribute to the advancement of medical research in the region. Embracing adaptability and fostering local partnerships will be essential as the regulatory environment evolves, ensuring that clinical trials remain robust and responsive to the needs of participants and stakeholders alike.
What is the primary authority overseeing clinical research approvals in Serbia?
The primary authority overseeing clinical research approvals in Serbia is the Medicines and Medical Devices Agency of Serbia (ALIMS).
What are the key regulations that researchers must be familiar with for adaptive trials in Serbia?
Researchers must be familiar with the Law on Medicines and Medical Devices and the Rulebook on Clinical Trials, which outline the necessary documentation, ethical considerations, and approval timelines.
What principles must adaptive design trial logistics in Serbia adhere to?
Adaptive design trial logistics in Serbia must adhere to Good Clinical Practice (GCP) principles.
Is prior approval required for modifications to the study design in adaptive trials?
Yes, any modifications to the study design in adaptive trials must receive prior approval from regulatory authorities.
How can engaging local regulatory experts benefit researchers in Serbia?
Engaging local regulatory experts, such as those at bioaccess, can streamline navigation of regulatory requirements and accelerate the approval process, enhancing the likelihood of successful outcomes.
What services does bioaccess offer to assist with clinical research in Serbia?
Bioaccess offers services including feasibility evaluations, examination and feedback on study documents, investigator selection, project management, and reporting.
How does collaboration with local experts impact clinical research studies in Serbia?
Collaboration with local experts like bioaccess is crucial for navigating regulatory complexities, effectively addressing challenges, and enhancing the chances of success in clinical research studies.