


Understanding data privacy rules in the context of drug trials is increasingly critical, particularly in Croatia, where stringent regulations govern the handling of personal information. This article explores the essential principles of data privacy that not only safeguard participant rights but also ensure compliance with both local and European laws. As the landscape of clinical research evolves, researchers must consider:
This inquiry is vital for fostering a responsible research environment.
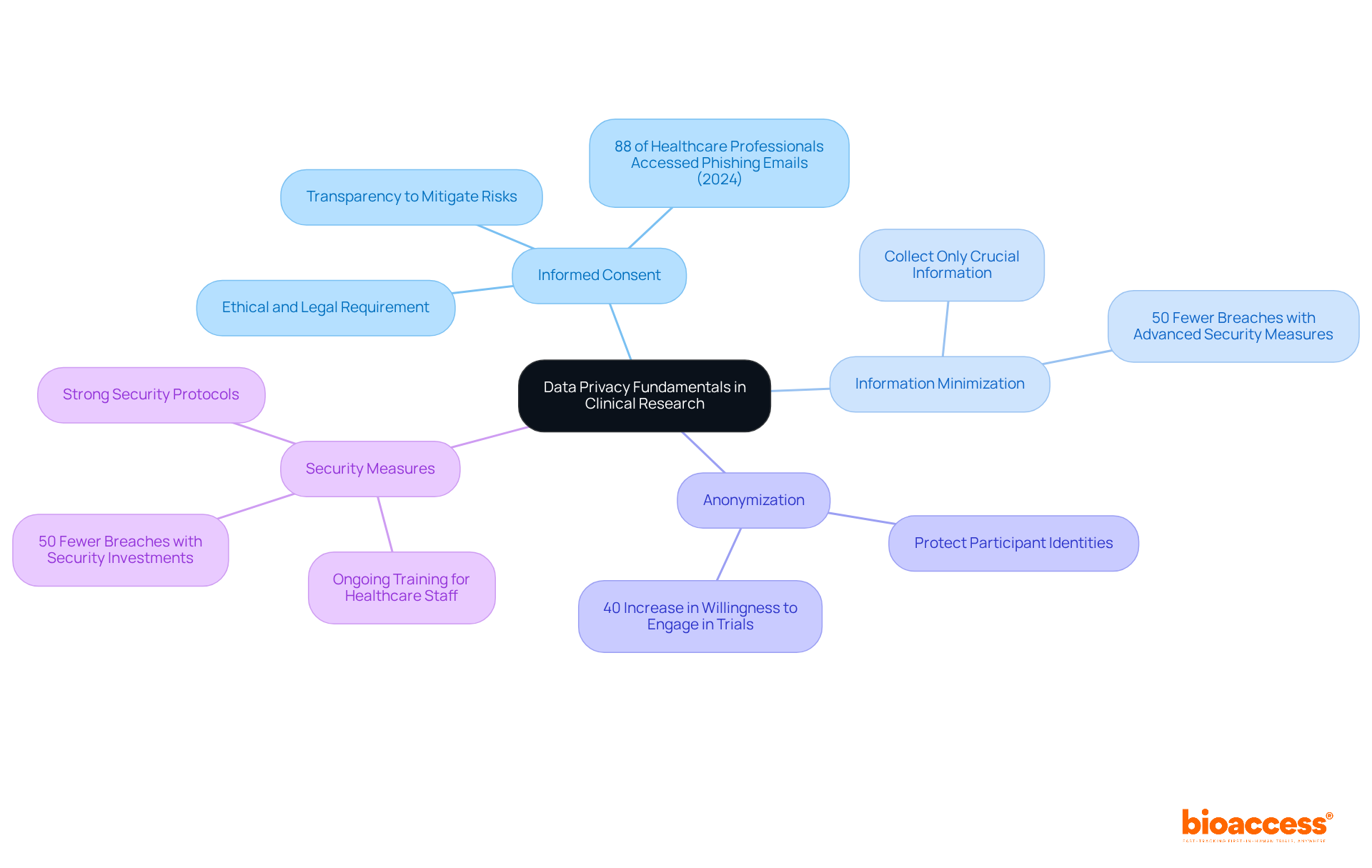
The data privacy rules for drug trials in Croatia are paramount, ensuring the protection of personal information collected from participants in clinical research. The key principles guiding this essential aspect include:
Informed Consent: Participants must be thoroughly informed about how their data will be utilized and must provide explicit consent for this use. This process is not only an ethical obligation but also a legal requirement under GDPR, local Croatian laws, and the data privacy rules for drug trials in Croatia. Notably, statistics reveal that 88% of healthcare professionals accessed phishing emails in 2024, underscoring the significance of transparency in the consent process to mitigate risks linked to breaches.
Information Minimization: Only the information crucial for the research should be gathered, significantly reducing the risk of exposure. For instance, a recent analysis indicates that healthcare organizations investing in advanced security measures experience 50% fewer breaches, highlighting the importance of minimizing data collection to enhance participant privacy.
Anonymization: Whenever feasible, information should be anonymized to protect participant identities. This practice not only adheres to GDPR but also fosters trust among participants. Studies indicate that anonymized information collection can enhance participant willingness to engage in clinical trials by up to 40%.
Security Measures: Strong security protocols are essential to safeguard information against unauthorized access. Recent reports suggest that healthcare organizations investing in advanced security measures experience 50% fewer breaches, emphasizing the critical need to prioritize information security in clinical research. Ongoing training for healthcare staff to detect and report malicious software and phishing emails is vital to maintaining these security measures.
These principles are integral to conducting ethical and compliant clinical trials while adhering to data privacy rules for drug trials in Croatia, ensuring that participant rights are respected and protected.
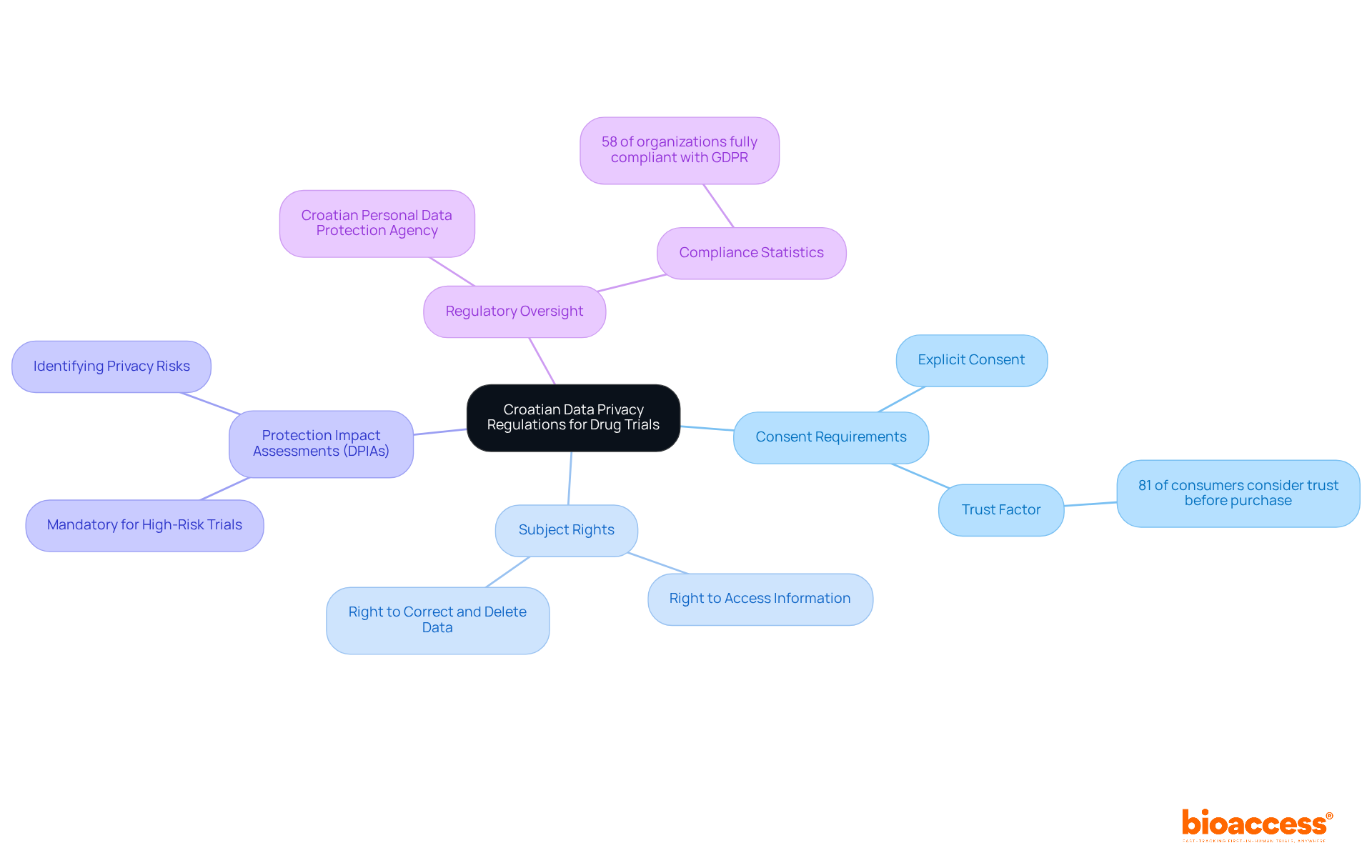
In Croatia, privacy regulations are primarily governed by the General Data Protection Regulation (GDPR) and the Croatian Act on Protection. It is crucial to understand the data privacy rules for drug trials in Croatia to ensure that clinical studies are conducted lawfully and ethically.
Consent Requirements: Explicit consent must be obtained from participants before processing their personal data. This is vital, as 81% of consumers now consider trust before making a purchase, emphasizing the significance of clear consent procedures in clinical studies.
Subject Rights: Participants possess the right to access, correct, and delete their information, which must be upheld throughout the study. This aligns with the GDPR's emphasis on empowering individuals regarding their personal information, particularly in the context of data privacy rules for drug trials in Croatia.
Protection Impact Assessments (DPIAs): Conducting DPIAs is mandatory for trials that may pose high risks to participants' rights and freedoms. This proactive approach helps identify and mitigate potential privacy risks, ensuring compliance with GDPR requirements.
Regulatory Oversight: The Croatian Personal Data Protection Agency oversees compliance and can impose penalties for violations. With only 58% of organizations fully compliant with GDPR, the role of this agency is essential in enforcing protection standards.
Furthermore, it is crucial to observe that 40% of organizations report AI-related confidentiality breaches, indicating changing risks in information security, particularly in clinical studies where AI might be employed. In an environment where 64% of consumers indicate choosing not to engage with companies because of confidentiality issues, understanding the data privacy rules for drug trials in Croatia is essential for maintaining trust and integrity in clinical research.
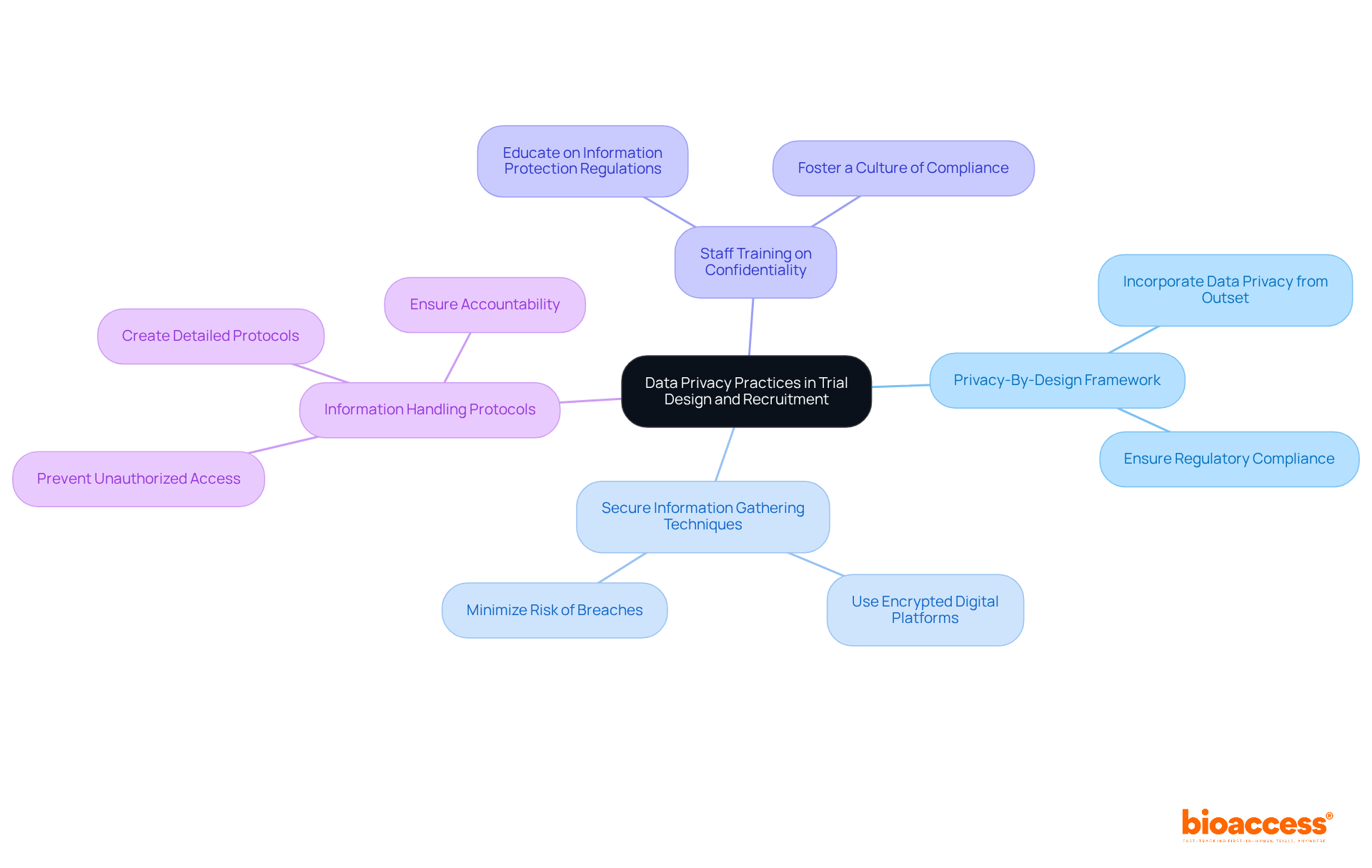
To implement data privacy practices in trial design and recruitment, consider the following strategies:
By proactively addressing information confidentiality, researchers can enhance participant confidence and streamline compliance.
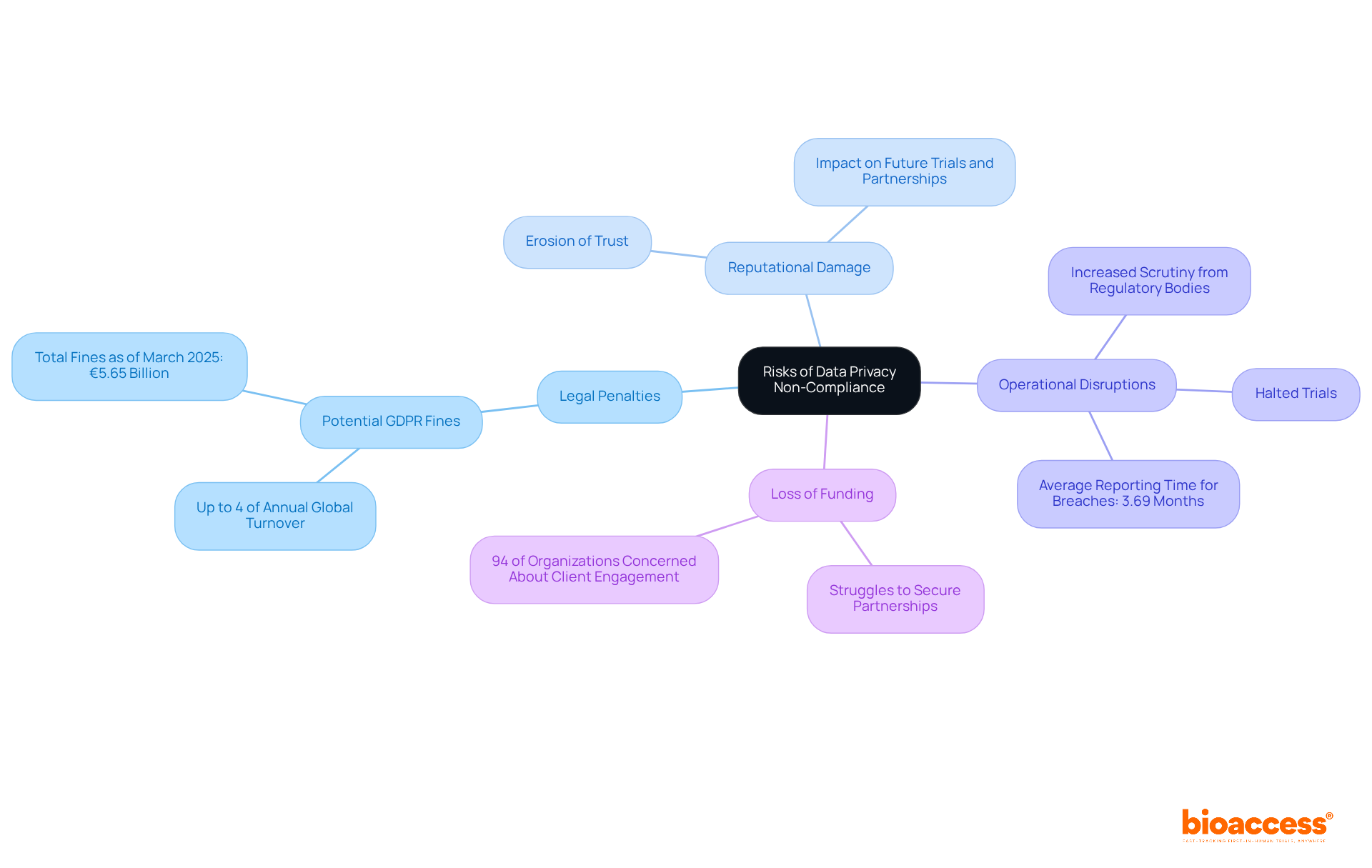
Non-compliance with data privacy regulations poses significant risks that organizations must take seriously.
Legal Penalties: Organizations can face hefty fines for violations, potentially reaching up to 4% of their annual global turnover under GDPR. As of March 2025, GDPR fines have totaled approximately €5.65 billion, highlighting the financial stakes involved in non-compliance.
Reputational Damage: Data breaches can severely damage an organization’s reputation, eroding trust among participants and stakeholders. High-profile breaches in the healthcare sector illustrate that organizations can suffer long-lasting reputational harm, which can hinder future trials and partnerships.
Operational Disruptions: Non-compliance may lead to halted trials, increased scrutiny from regulatory bodies, and potential legal actions from affected participants. The average time to report breaches in healthcare is 3.69 months, showcasing the operational challenges organizations face when navigating compliance issues.
Loss of Funding: Organizations with a history of non-compliance often struggle to secure funding or partnerships. Investors and stakeholders are increasingly cautious, with 94% of organizations indicating that clients would cease engagement if their information isn’t adequately protected.
Recognizing these risks allows researchers to prioritize compliance with data privacy rules for drug trials in Croatia, ensuring that their trials are conducted ethically and legally. This commitment not only safeguards the organization’s integrity but also opens doors to future opportunities.
Understanding the data privacy rules for drug trials in Croatia is essential for conducting ethical and compliant clinical research. Safeguarding personal information not only aligns with legal mandates but also fosters trust between researchers and participants. By adhering to principles such as informed consent, information minimization, anonymization, and robust security measures, researchers can ensure that participant rights are respected and protected throughout the trial process.
Key insights from the article highlight the importance of:
The Croatian Personal Data Protection Agency plays a crucial role in ensuring regulatory oversight and maintaining compliance. The financial and reputational risks associated with non-compliance, including potential legal penalties and operational disruptions, serve as a stark reminder of the stakes involved in upholding data privacy standards.
In conclusion, prioritizing data privacy in clinical trials is not merely a regulatory obligation; it is a critical component of responsible research that significantly impacts participant engagement and trust. By implementing best practices and fostering a culture of compliance, researchers can protect participant information and contribute to the integrity and credibility of the clinical research landscape in Croatia. Engaging with these principles is vital for ensuring that the future of drug trials remains ethical, transparent, and respectful of individual rights.
What are the key principles of data privacy in clinical research in Croatia?
The key principles include Informed Consent, Information Minimization, Anonymization, and Security Measures. These principles ensure the protection of personal information collected from participants.
What is the importance of Informed Consent in clinical trials?
Informed Consent is crucial as participants must be fully informed about how their data will be used and provide explicit consent. This is both an ethical obligation and a legal requirement under GDPR and local Croatian laws.
How does Information Minimization contribute to data privacy?
Information Minimization involves collecting only the data essential for the research, which significantly reduces the risk of exposure and enhances participant privacy.
What role does Anonymization play in protecting participant identities?
Anonymization helps protect participant identities by ensuring that personal information is not linked to individuals. This practice adheres to GDPR and can increase participant willingness to engage in clinical trials by up to 40%.
What security measures are necessary for safeguarding participant data?
Strong security protocols are essential to protect information from unauthorized access. Organizations that invest in advanced security measures experience 50% fewer breaches, highlighting the importance of prioritizing information security.
Why is ongoing training for healthcare staff important in clinical research?
Ongoing training is vital for healthcare staff to detect and report malicious software and phishing emails, which helps maintain security measures and protect participant data effectively.