


Navigating the intricate landscape of early feasibility trial submissions under Malmed demands a nuanced understanding of regulatory requirements-elements that can significantly influence a study's success. Researchers can gain invaluable insights into best practices that not only streamline the approval process but also enhance the ethical integrity and scientific validity of their trials.
With the stakes so high-where delays can cost sponsors millions and impede recruitment efforts-how can researchers ensure they meet these stringent guidelines while fostering diversity and maintaining clear communication with regulatory authorities? This article aims to explore these critical challenges and provide actionable strategies for success.
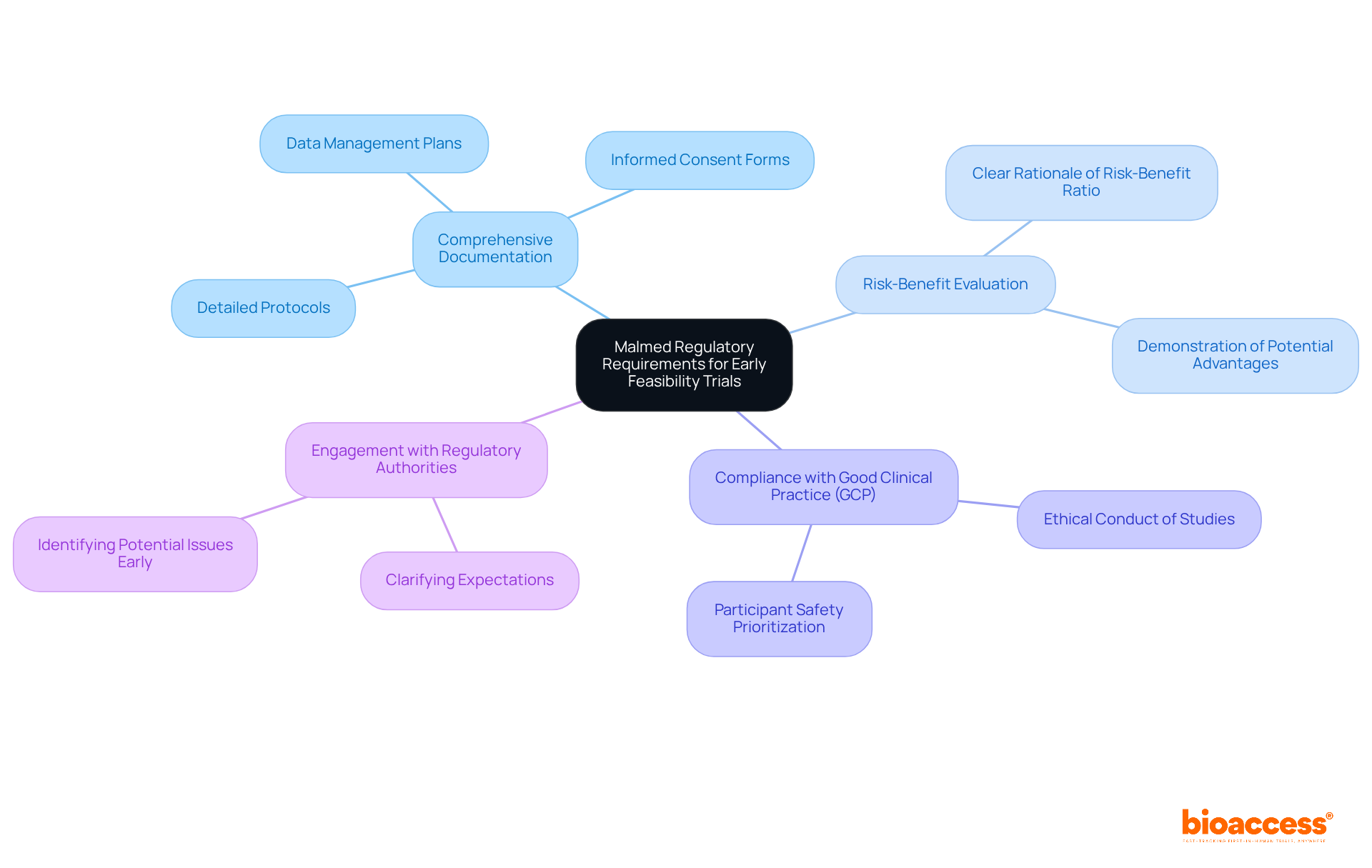
To successfully navigate early feasibility trial submissions under Malmed, it’s crucial to grasp the regulatory landscape. Malmed, the Agency for Medicines and Medical Devices in North Macedonia, has specific guidelines governing the submission and execution of clinical studies. Key requirements include:
Utilizing bioaccess's extensive clinical study management services can significantly enhance the viability of your studies. Bioaccess specializes in feasibility studies, site selection, compliance reviews, setup, import permits, project management, and reporting, ensuring meticulous handling of all study aspects. Recognizing the financial implications of delays in clinical studies-costing sponsors between $600,000 and $8 million for each day a study is postponed-is essential. Moreover, around 80% of clinical studies face delays or closures due to recruitment challenges, underscoring the importance of comprehensive documentation and proactive engagement with regulatory bodies.
By understanding these requirements and leveraging bioaccess's expert services, researchers can boost their chances of obtaining timely approvals and conducting successful experiments.
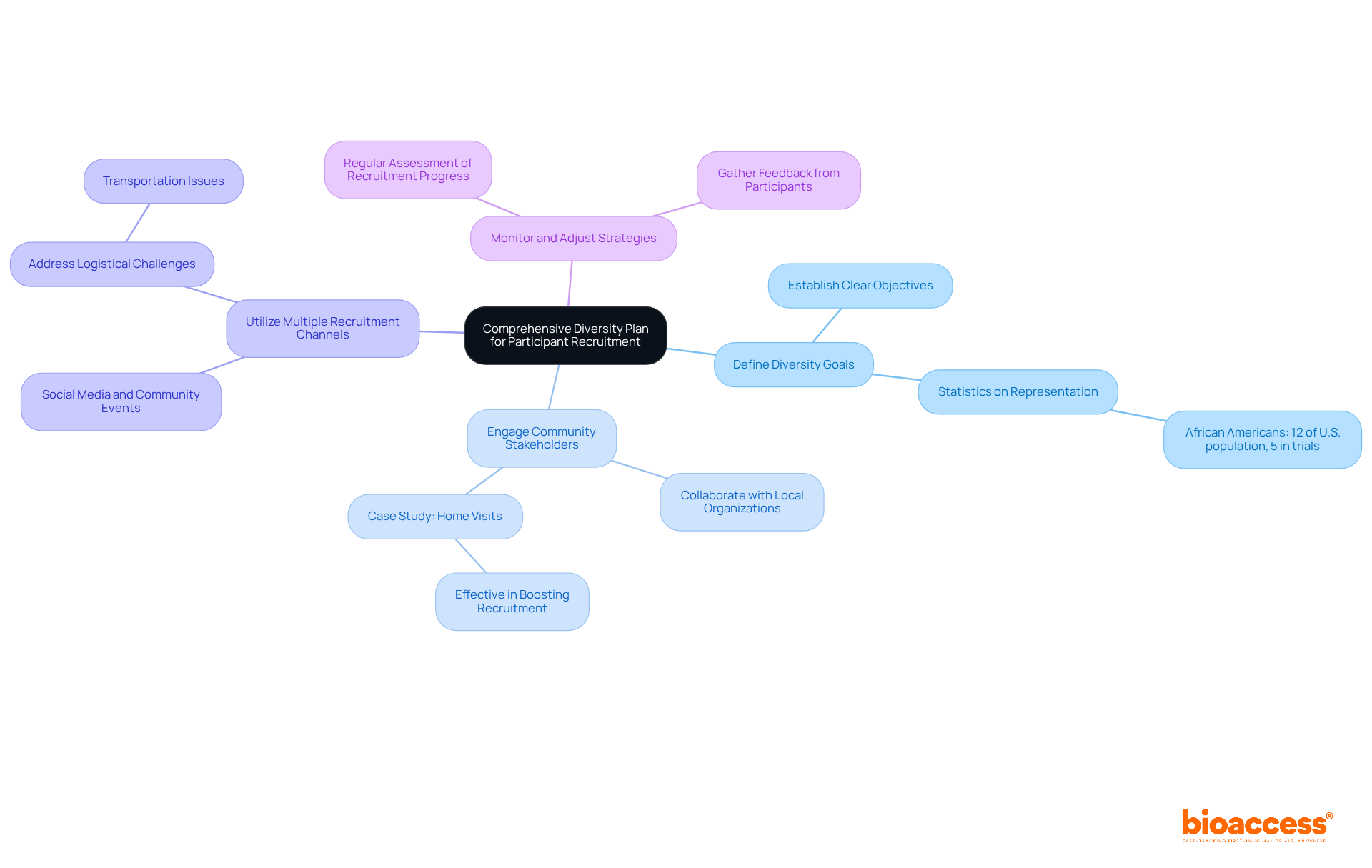
A robust diversity strategy is crucial for ensuring that early feasibility tests accurately reflect the demographics of the broader population. To achieve this, consider the following key components:
Define Diversity Goals: Establish clear objectives for demographic representation, encompassing age, gender, ethnicity, and health status. This clarity aids in setting measurable recruitment targets. For example, while African Americans make up 12% of the U.S. population, they represent only 5% of those involved in clinical trials. This stark contrast highlights the urgent need to address diversity in clinical research.
Engage Community Stakeholders: Collaborate with local organizations and community leaders to foster trust and enhance outreach efforts. Such partnerships can significantly improve recruitment and ensure that studies are culturally sensitive. A notable case study involves home visits, which have proven effective in boosting recruitment rates among underrepresented populations, showcasing the power of tailored engagement strategies.
Utilize Multiple Recruitment Channels: Implement a diverse array of recruitment strategies, including social media, community events, and partnerships with healthcare providers, to reach a wider audience. It's essential to tackle logistical challenges, such as transportation issues faced by potential participants, to improve access and involvement.
Monitor and Adjust Strategies: Regularly assess recruitment progress and be ready to modify strategies as necessary to meet diversity goals. This process may include analyzing recruitment data and gathering feedback from participants. Recognizing the historical mistrust of medical research within minority communities is vital for building trust and ensuring effective engagement.
By applying these strategies, you not only fulfill compliance requirements but also enrich the research landscape by incorporating diverse perspectives, ultimately leading to more effective and applicable outcomes.
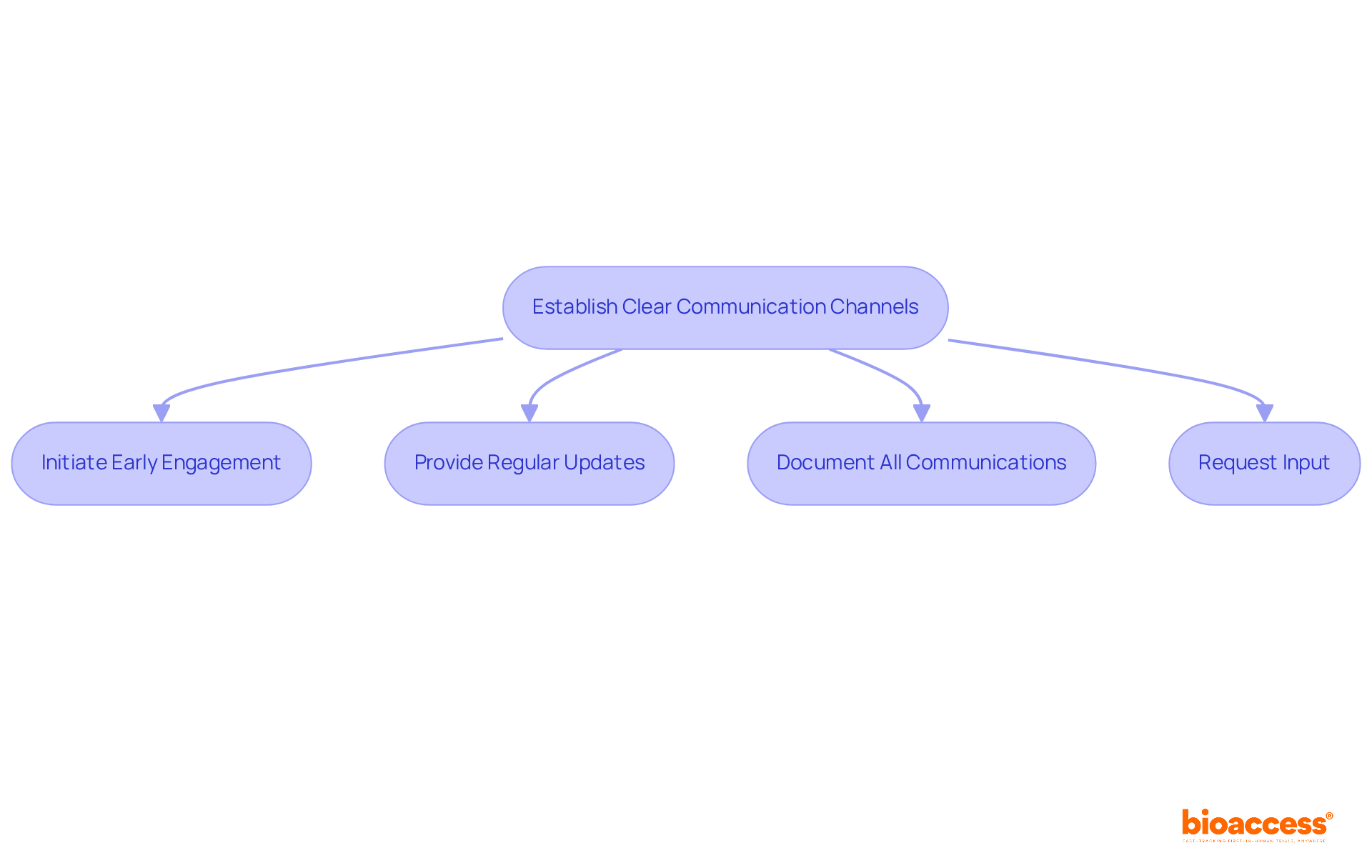
Effective communication with oversight bodies is crucial for the prompt approval of early feasibility trial submissions under malmed. To establish and maintain these vital channels, consider the following best practices:
By applying these communication strategies, researchers can foster strong connections with regulatory authorities. This not only facilitates smoother interactions but also improves the overall success of their studies.
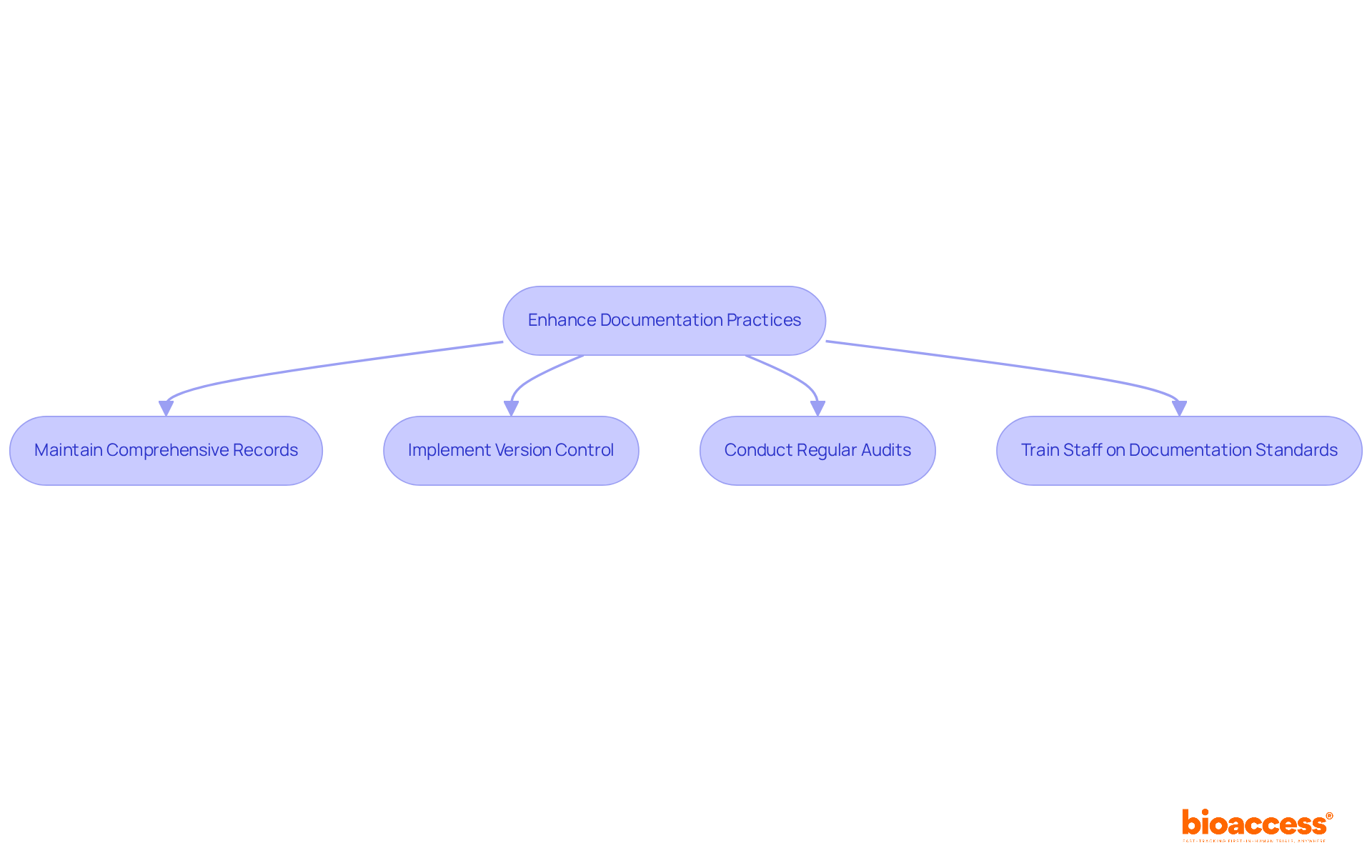
Meticulous documentation is essential for the success of early feasibility trial submissions under malmed in clinical research. It not only ensures compliance but also upholds ethical integrity. Here are key practices that can significantly enhance your documentation efforts:
Maintain Comprehensive Records: Document every aspect of the trial, including study protocols, informed consent forms, and subject data. This practice guarantees that all information is readily accessible for review and audit, supporting compliance with regulations and ensuring participant safety.
Implement Version Control: Utilize version control for all documents to track changes and ensure that the most current versions are submitted to governing bodies. This method minimizes confusion and mistakes, improving the clarity of submissions and facilitating smoother interactions with authorities.
Conduct Regular Audits: Periodically review documentation practices to ensure adherence to regulatory standards and internal protocols. This proactive strategy helps identify and rectify potential issues before they escalate, thereby maintaining the integrity of the process and ensuring compliance with ethical guidelines.
Train Staff on Documentation Standards: Provide comprehensive training for all team members on the importance of accurate documentation and ethical compliance. This fosters a culture of accountability and ensures that everyone understands their responsibilities, ultimately enhancing the quality and reliability of the data.
By adhering to these documentation practices, researchers can bolster the credibility of their early feasibility trial submissions under malmed and significantly enhance the overall success of their clinical trials.
Navigating early feasibility trial submissions under Malmed demands a thorough grasp of the regulatory framework and established best practices. By emphasizing clear documentation, conducting risk-benefit evaluations, and adhering to Good Clinical Practice (GCP), researchers can streamline their submission processes and significantly boost their chances of securing timely approvals. Engaging with regulatory authorities from the outset and fostering open communication channels further simplifies the submission process, allowing for proactive resolution of potential challenges.
A critical strategy involves crafting a robust diversity plan for participant recruitment. This not only meets compliance requirements but also enriches the research landscape. Engaging community stakeholders, leveraging diverse recruitment channels, and continuously monitoring and refining strategies are vital steps toward achieving meaningful demographic representation in clinical trials. Moreover, meticulous documentation and ethical compliance are essential for upholding the integrity of the research process.
Ultimately, embracing these best practices not only facilitates successful early feasibility trial submissions under Malmed but also advances the broader objective of enhancing clinical research. By promoting collaboration, increasing diversity, and ensuring rigorous documentation, researchers can profoundly influence the effectiveness and relevance of their studies, paving the way for innovations that genuinely address the needs of diverse populations.
What is Malmed and its role in early feasibility trials?
Malmed is the Agency for Medicines and Medical Devices in North Macedonia, responsible for regulating the submission and execution of clinical studies, including early feasibility trials.
What documentation is required for early feasibility trial submissions under Malmed?
All applications must include detailed protocols, informed consent forms, and data management plans to ensure the study is ethically sound and scientifically valid.
What is the significance of the risk-benefit evaluation in trial submissions?
A clear rationale of the risk-benefit ratio must be presented, demonstrating that the potential advantages to individuals exceed the associated risks.
What guidelines must be followed during early feasibility trials?
Compliance with Good Clinical Practice (GCP) guidelines is mandatory, ensuring that studies are conducted ethically and that participant safety is prioritized.
How can engagement with regulatory authorities benefit early feasibility trial submissions?
Engaging with regulatory authorities can clarify expectations and streamline the approval process, helping to identify potential issues before they arise.
What services does Bioaccess offer to enhance clinical studies?
Bioaccess specializes in feasibility studies, site selection, compliance reviews, setup, import permits, project management, and reporting, ensuring meticulous handling of all study aspects.
What are the financial implications of delays in clinical studies?
Delays can cost sponsors between $600,000 and $8 million for each day a study is postponed, highlighting the importance of timely approvals.
What challenges do clinical studies often face?
Around 80% of clinical studies encounter delays or closures due to recruitment challenges, emphasizing the need for comprehensive documentation and proactive engagement with regulatory bodies.