

Understanding the dynamics of ethics committees is crucial for researchers navigating the complex landscape of clinical trials. These committees uphold the ethical standards that protect participants and significantly influence the efficiency of the research approval process. Yet, many researchers face challenges in optimizing meeting frequencies and timelines, often leading to delays and complications in their studies.
How can researchers effectively manage these timelines to ensure compliance and timely approvals, ultimately enhancing the success of their clinical research endeavors?
Ethics panels, known as Institutional Review Boards (IRBs), play a pivotal role in overseeing clinical studies, ensuring that ethical standards are upheld. Comprising a diverse group of members-including healthcare professionals, ethicists, and community representatives-these committees are essential in evaluating the moral implications of proposed research. Their primary responsibilities include:
The makeup of ethics committees is designed to reflect a broad spectrum of expertise, with many groups including at least one member from the non-scientific community. This diversity enriches the decision-making process, ensuring that various perspectives are taken into account. Research shows that 33% of studies considered 'user satisfaction' as a metric, with 94% reporting a positive perception of the impact of Clinical Ethics Committees (CECs), which is crucial for building trust and credibility in clinical research.
Understanding the structure and functions of ethics panels, as well as the ethics committee timelines and meeting frequency, is essential for researchers aiming to engage effectively with these organizations. This knowledge ultimately facilitates smoother approval processes and enhances the ethical conduct of clinical trials.

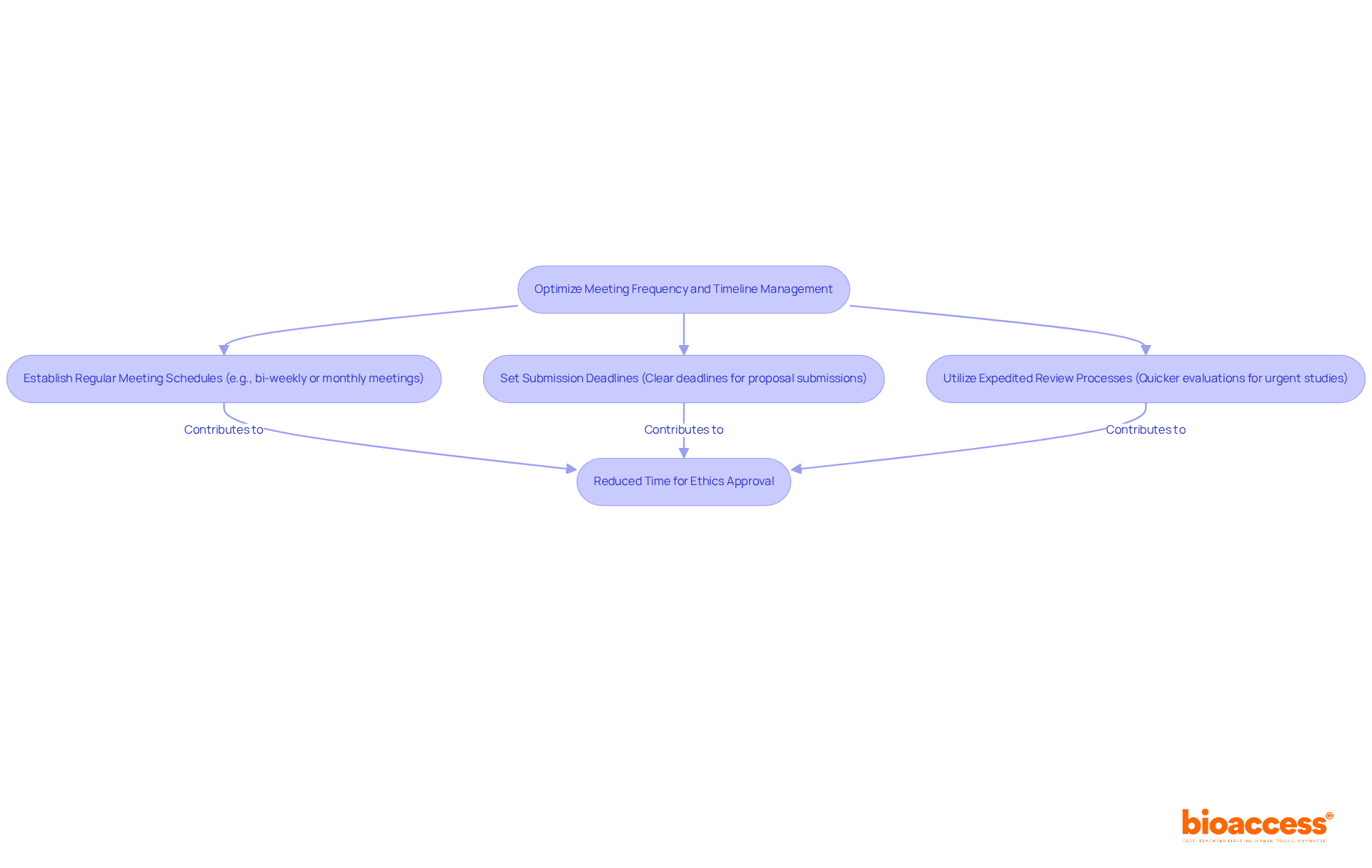
To ensure timely reviews and approvals, optimizing the ethics committee timelines and meeting frequency of ethics groups is crucial. Here are some best practices:
By adhering to these practices, researchers can significantly reduce the time required to obtain ethics approval, thus positively impacting ethics committee timelines and meeting frequency, and accelerating their clinical research.
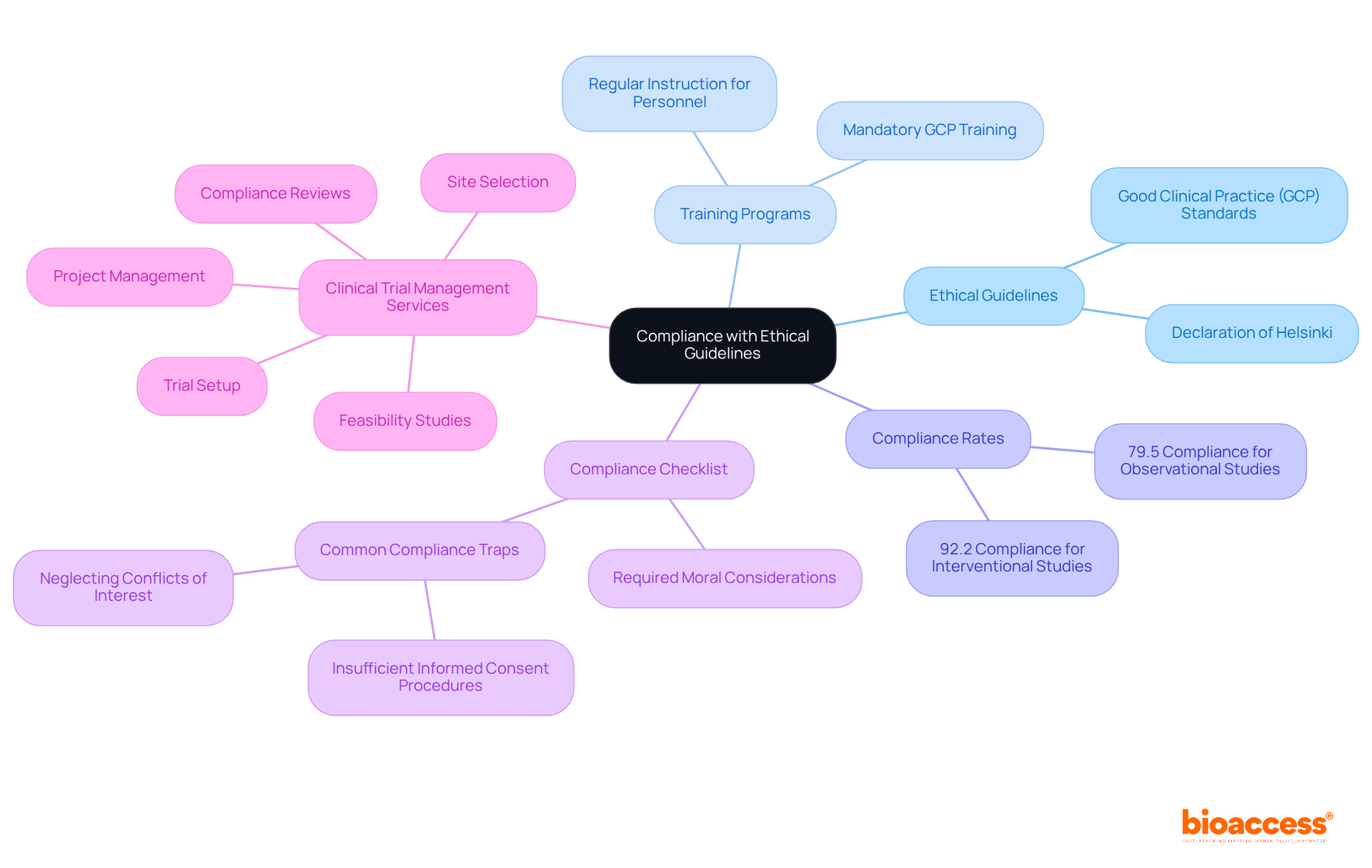
Ensuring adherence to moral guidelines and regulations is crucial for the success of any clinical trial. Researchers must be well-acquainted with both local and international ethical guidelines, notably the Declaration of Helsinki and Good Clinical Practice (GCP) standards. This foundational knowledge is vital for designing compliant studies that prioritize participant welfare. The 2000 update of the Declaration highlights the necessity for moral assessment and informed consent to safeguard participants in studies, establishing a minimum set of international standards binding on physicians globally.
Regular instruction for personnel on moral standards significantly reinforces the importance of these guidelines. Effective training programs improve awareness of responsibilities and moral duties, fostering a culture of compliance within research teams. Notably, GCP compliance rates for interventional studies average 92.2%, while observational studies show a lower compliance rate of 79.5%. This highlights the critical need for ongoing education and training.
Creating a thorough compliance checklist that details all required moral considerations serves as a practical tool for researchers. This checklist ensures that proposals meet required standards before submission, taking into account the ethics committee timelines and meeting frequency, thus streamlining the approval process. Common traps in moral compliance include insufficient informed consent procedures and neglect to reveal possible conflicts of interest, which can threaten the integrity of the study.
In addition to these strategies, bioaccess offers comprehensive clinical trial management services that include feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting. By prioritizing adherence to moral standards, researchers not only protect participants but also enhance the credibility and acceptance of their findings. As the World Medical Association indicates, ethical duties must take precedence over legal commitments when conflicts occur, highlighting the significance of upholding high ethical standards in clinical studies.
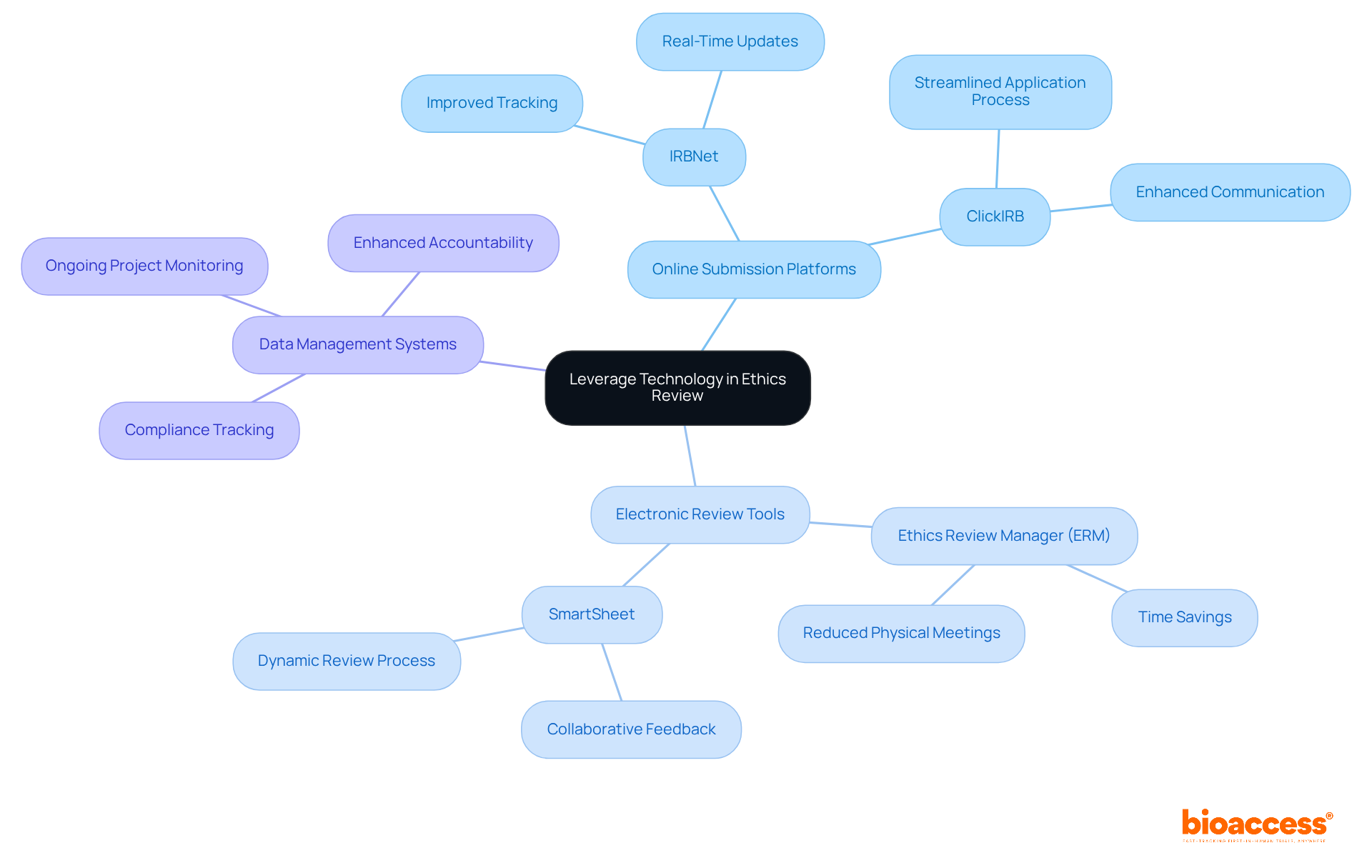
Incorporating technology into the ethics review process can significantly enhance efficiency and transparency. This integration is not just a trend; it’s a necessity for modern clinical research. Here are some best practices that can transform your approach:
Utilize Online Submission Platforms: Implementing digital platforms for submitting research proposals streamlines the application process. This allows for easier tracking and management of submissions. For instance, many institutions now leverage online systems like IRBNet and ClickIRB, which facilitate real-time updates and communication, ultimately improving overall workflow.
Adopt Electronic Review Tools: Tools that enable committee members to review documents electronically, such as Ethics Review Manager (ERM) and SmartSheet, can save time and reduce the need for physical meetings. These tools foster collaborative feedback, making the review process more dynamic and responsive to researchers' needs.
Implement Data Management Systems: Utilizing data management systems assists in tracking compliance and monitoring ongoing projects. This ensures that all ethical standards are continuously met throughout the investigation's lifecycle. Such a proactive approach not only enhances accountability but also upholds the integrity of the investigation process.
While leveraging technology can lead to more efficient operations, it’s crucial to address challenges related to data privacy and accuracy, as highlighted in recent studies. By thoughtfully incorporating these technologies, ethics committee timelines and meeting frequency can be improved, ultimately leading to faster and more effective reviews of clinical research proposals.
Mastering the complexities of ethics committee timelines and meeting frequency is crucial for researchers striving for success in clinical trials. These committees uphold ethical standards and facilitate a smoother approval process, ensuring that the rights and welfare of participants are prioritized. By understanding their structure and functions, optimizing meeting schedules, and leveraging technology, researchers can significantly enhance the efficiency of the review process.
Key insights from this discussion highlight the necessity of:
Furthermore, ensuring compliance with ethical guidelines and incorporating technology into the review process can streamline operations, leading to quicker approvals and greater accountability. The diverse composition of ethics committees enriches their decision-making, ultimately fostering trust and credibility in clinical research.
As the landscape of clinical trials evolves, it is vital for researchers to prioritize these best practices. By doing so, they not only enhance the ethical conduct of their studies but also contribute to advancing research that genuinely respects and protects participant rights. Embracing these strategies will lead to successful outcomes and set a standard for ethical excellence in the field.
What is the role of Ethics Committees in clinical studies?
Ethics Committees, also known as Institutional Review Boards (IRBs), oversee clinical studies to ensure ethical standards are upheld, protecting the rights and welfare of research participants.
What are the primary responsibilities of Ethics Committees?
Their primary responsibilities include reviewing research proposals, monitoring ongoing research, and providing guidance on ethical dilemmas that may arise during the research process.
How do Ethics Committees review research proposals?
Committees thoroughly examine the ethical dimensions of research proposals to ensure compliance with ethical standards, protecting participants from issues like informed consent violations and safety concerns.
What is the significance of monitoring ongoing research?
Continuous monitoring by Ethics Committees is vital for ensuring compliance with ethical standards and addressing any emerging concerns, thus safeguarding participants and maintaining the integrity of the research.
How do Ethics Committees provide guidance to researchers?
They offer invaluable advice on ethical dilemmas that researchers may face, helping them navigate complex moral challenges, especially in studies involving vulnerable populations.
What is the composition of Ethics Committees?
Ethics Committees are composed of a diverse group of members, including healthcare professionals, ethicists, community representatives, and at least one member with experience working with vulnerable groups.
Why is diversity important in Ethics Committees?
Diversity enriches the decision-making process by ensuring various perspectives are considered, which is crucial for ethical evaluations and building trust in clinical research.
What impact do Clinical Ethics Committees (CECs) have on research?
Research indicates that 94% of studies report a positive perception of the impact of CECs, highlighting their importance in enhancing user satisfaction and credibility in clinical research.
Why is it important for researchers to understand Ethics Committees?
Understanding the structure, functions, timelines, and meeting frequency of Ethics Committees helps researchers engage effectively with these organizations, facilitating smoother approval processes and promoting ethical conduct in clinical trials.