


Understanding the nuances of informed consent is essential for researchers working under Macedonian law, as it acts as a crucial safeguard for participant rights. This guide explores the legal requirements and best practices for informed consent documentation, paving the way for ethical clinical research. As researchers navigate the complexities of compliance, they may ask themselves: how can they ensure that consent not only meets legal standards but also fosters genuine understanding and trust among participants?
Informed consent is not just a legal obligation; it is a cornerstone of ethical research. By prioritizing transparency and clarity, researchers can build a foundation of trust with their participants. This guide aims to illuminate the path toward achieving this balance, ensuring that consent processes are both legally sound and ethically robust.
Knowledgeable agreement is a cornerstone of clinical research, ensuring that individuals fully understand the nature, risks, and benefits of the study they are considering. In Macedonia, informed consent documentation under Macedonian law is essential, as it is not merely a procedural formality but a legal requirement designed to protect individuals' rights. This process mandates that researchers provide clear and comprehensive information, empowering individuals to make informed decisions about their participation.
The significance of knowledgeable approval extends beyond mere compliance with legal standards; it fosters trust and transparency in the research process, which is essential for ethical clinical practice. By grasping and adhering to these legal requirements, such as informed consent documentation under Macedonian law, researchers can bolster participant confidence and cultivate a more ethical research environment. This commitment to ethical standards not only enhances the integrity of the research but also promotes a culture of respect and responsibility within the clinical research community.
In Macedonia, the legal framework governing informed consent is primarily established by the Law on the Protection of Patients' Rights and the Law on Personal Data Protection. Researchers must adhere to several key requirements to ensure that consent is valid:
Moreover, it is essential for researchers to acknowledge that individuals maintain the right to retract their agreement at any moment without experiencing adverse consequences. Familiarizing oneself with these legal stipulations is vital for conducting ethical and compliant research in Macedonia.
Case studies, including those involving vulnerable groups, emphasize the significance of these principles. They demonstrate that clear communication and respect for autonomy are vital in building trust and integrity in clinical research. For instance, the requirement for discussions regarding informed consent to occur in private environments is supported by external sources, which stress the importance of clear communication and enabling individuals to inquire and reflect on their involvement thoroughly. This approach not only aligns with legal requirements but also enhances the ethical standards of research practices.
When drafting informed consent documentation under Macedonian law, researchers must adhere to best practices that ensure clarity and comprehension, which are crucial in clinical research.
Use Clear Language: It's essential to avoid technical jargon and write at a reading level accessible to the target population. Research indicates that consent forms should ideally be understandable at an 8th-grade reading level to enhance comprehension among diverse individuals. Alarmingly, the median Flesch-Kincaid Reading Grade Level of all Research Informed Consent Forms (RICFs) was 10.1, underscoring current readability challenges that must be addressed.
Include Essential Elements: Clearly outlining the study's purpose, procedures, potential risks, benefits, and the right to withdraw is vital. This clarity not only adheres to ethical standards but also empowers individuals to make informed choices. Notably, studies reveal that 84% of informed consent forms written in English were difficult to read, emphasizing the importance of clear language.
Format for Readability: To enhance readability, utilize headings, bullet points, and short paragraphs. Well-organized documents significantly improve participant engagement and understanding, thereby reducing the likelihood of consent refusal.
Provide Contact Information: Including details for individuals to reach out with questions or concerns fosters trust and encourages open communication, which is essential for ethical research practices.
Review and Revise: It is crucial to have the document reviewed by legal and ethical boards to ensure compliance with Macedonian laws. Regular updates based on feedback can help maintain clarity and relevance, addressing any challenges that may arise in the consent process. As Michael K. Paasche-Orlow emphasizes, important documents for patients must be written in clear, direct language.
By adhering to these guidelines, researchers can create effective consent documents that not only meet regulatory requirements but also promote informed decision-making among participants.
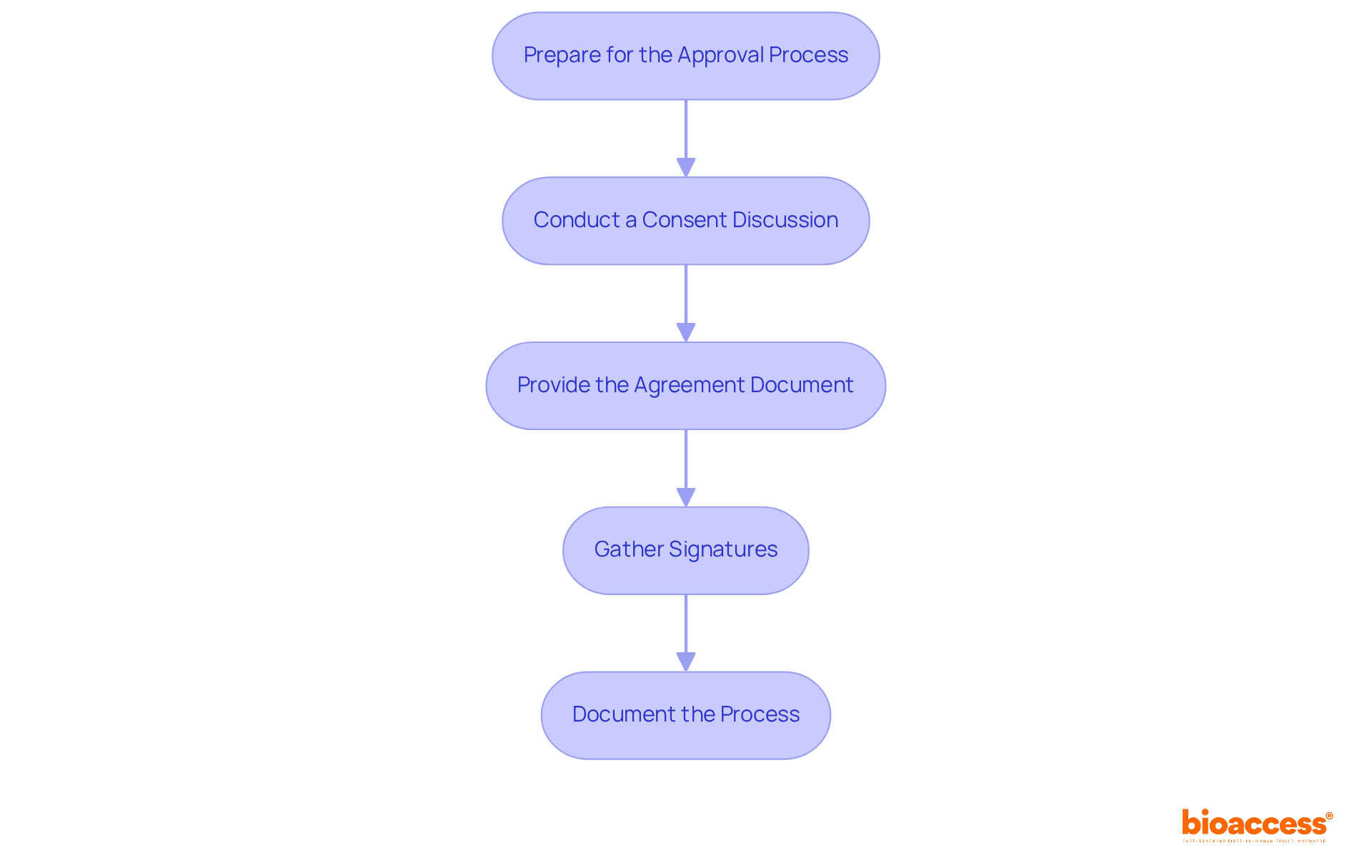
To effectively obtain and document informed consent, researchers must follow these essential steps:
Prepare for the Approval Process: It’s vital that all team members are well-trained in the informed authorization procedure and have a comprehensive understanding of the research details. This foundational knowledge fosters trust and transparency; inadequate training can lead to misunderstandings. As Lika Nusbaum points out, enhancing education and training for research staff is crucial for effective communication during the consent process.
Conduct a Consent Discussion: Engage individuals in a meaningful dialogue about the research, encouraging them to ask questions and voice any concerns. This interaction is critical; studies show that 74.7% of individuals understood the study's nature and the voluntary aspect of participation when actively engaged. Additionally, 63.5% of phase I trial patients had no therapeutic misconceptions, underscoring the necessity of clear communication.
Provide the Agreement Document: Present the awareness agreement document clearly, allowing individuals ample time to read and comprehend its contents. This step is crucial for ensuring that participants are fully informed, especially about informed consent documentation under Macedonian law; 75.8% of individuals understood their right to withdraw at any time when adequately informed. Furthermore, half of those involved in clinical trials grasped the elements of informed consent, highlighting the importance of effective communication.
Gather Signatures: Have individuals sign the agreement form, ensuring they receive a copy for their records. This not only formalizes the agreement but also reinforces their understanding of the study's implications.
Document the Process: Meticulously record the date and specifics of the consent discussion, including any questions raised by participants. This informed consent documentation under Macedonian law is essential for adhering to ethical standards and regulatory requirements, as it demonstrates accountability and respect for individual autonomy. Notably, 54.3% of individuals in developing nations had no treatment misunderstandings, compared to 85.2% in least developed countries, highlighting the need for thorough documentation and understanding.
By adhering to these best practices, researchers can significantly enhance the informed consent process, ultimately improving volunteer understanding and engagement in clinical trials.
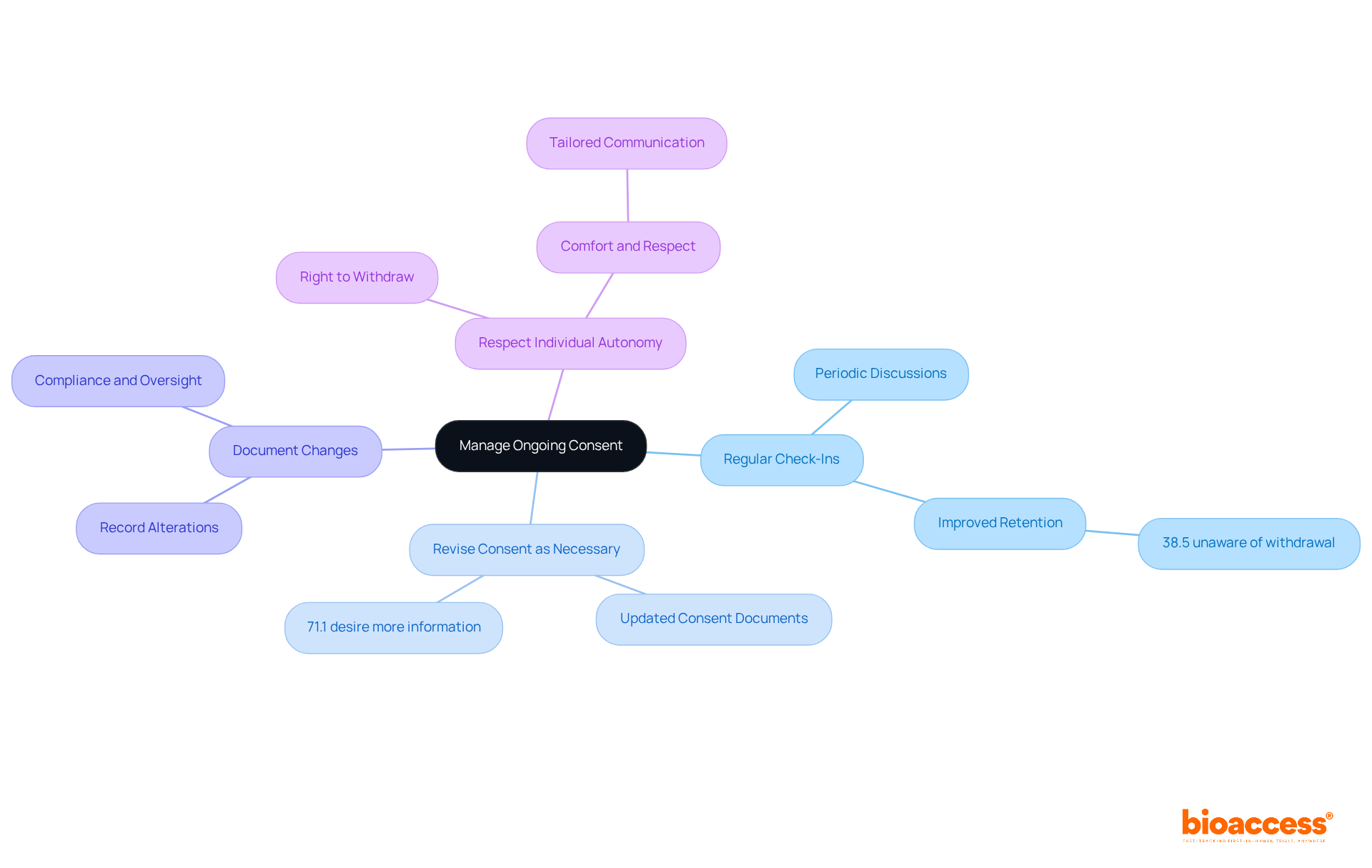
Managing ongoing approval is essential for maintaining ethical standards in clinical research. Key practices include:
Regular Check-Ins: Schedule periodic discussions with attendees to reaffirm their consent and address any new questions or concerns. This proactive strategy can significantly improve attendee retention. Research suggests that regular involvement fosters trust and transparency. Notably, 38.5% of participants at follow-up indicated feeling 'not at all' aware of the possibility of withdrawal, emphasizing the necessity for consistent communication.
Revise Consent as Necessary: If there are substantial alterations to the study protocol or related risks, provide individuals with revised consent documents. This ensures they are fully informed and can make decisions based on the most current information related to informed consent documentation under Macedonian law. The finding that 71.1% of respondents at follow-up expressed a desire for more information highlights the significance of keeping individuals informed.
Document Changes: Maintain detailed records of any alterations in approval status, including dates and reasons for re-approval. This documentation is vital for compliance and ethical oversight, reflecting the dynamic nature of involvement throughout the study.
Respect Individual Autonomy: Prioritize the individual's right to withdraw consent at any time. Ensuring that individuals feel comfortable and respected throughout the research process is crucial for ethical integrity. Research has shown that individuals who are consistently updated about their entitlements and the project's progress are more likely to stay engaged and satisfied with their involvement. Moreover, the challenges faced by participants from developing nations in understanding informed consent documentation under Macedonian law highlight the need for tailored communication approaches.
By actively managing ongoing consent, researchers can uphold ethical standards while enhancing the overall quality and credibility of their studies.
Informed consent documentation stands as a cornerstone of ethical clinical research in Macedonia, transcending mere procedural necessity. By grasping and adhering to the legal requirements and best practices detailed in this guide, researchers can ensure that participants are not only fully informed but also respected and empowered to make their own decisions regarding study participation. This unwavering commitment to ethical standards is essential for fostering trust and transparency within the research community.
Key points discussed encompass the legal framework governing informed consent, which underscores the importance of clarity, voluntary participation, and the right to withdraw at any time. Crafting effective consent documents demands careful attention to language and format to enhance comprehension. Moreover, the ongoing management of consent throughout the research process ensures that participants remain informed and engaged. By implementing these practices, researchers can significantly bolster participant understanding and cultivate a culture of respect and responsibility.
Ultimately, the significance of informed consent extends far beyond legal compliance; it safeguards individual rights and enhances the integrity of clinical research. Researchers are urged to prioritize informed consent as a vital component of their studies, recognizing its pivotal role in promoting ethical practices and participant empowerment. By doing so, they contribute to a more trustworthy and responsible research environment in Macedonia.
What is the importance of informed consent in Macedonian law?
Informed consent is a cornerstone of clinical research in Macedonia, ensuring individuals fully understand the nature, risks, and benefits of a study. It is a legal requirement designed to protect individuals' rights and fosters trust and transparency in the research process.
What legal frameworks govern informed consent in Macedonia?
The legal framework for informed consent in Macedonia is primarily established by the Law on the Protection of Patients' Rights and the Law on Personal Data Protection.
What are the key requirements for valid informed consent in Macedonia?
The key requirements for valid informed consent include: - Freely Given: Participants must provide consent voluntarily without coercion. - Specific: The consent must clearly outline the specific research and nature of involvement. - Informed: Participants should receive comprehensive information about the study's purpose, procedures, risks, and benefits. - Clear: The consent document must be straightforward and easily understandable.
Can participants retract their consent in Macedonia?
Yes, individuals have the right to retract their consent at any time without facing adverse consequences.
Why is clear communication important in the informed consent process?
Clear communication is vital for building trust and integrity in clinical research. It allows participants to inquire and reflect on their involvement, which enhances the ethical standards of research practices.
How do case studies emphasize the principles of informed consent?
Case studies, particularly those involving vulnerable groups, highlight the significance of clear communication and respect for autonomy in the informed consent process, reinforcing the need for ethical and compliant research practices.