


Navigating the complexities of clinical research demands a profound understanding of regulatory frameworks, especially when it comes to managing Contract Research Organizations (CROs) in TGA-compliant studies. The Therapeutic Goods Administration (TGA) enforces stringent guidelines that dictate the regulation of therapeutic products in Australia. Compliance is not merely a formality; it is a cornerstone of successful research outcomes. This article explores best practices for managing CROs, emphasizing:
These practices can significantly enhance the integrity and success of clinical trials. How can researchers ensure their CRO management practices align with TGA standards while maximizing the potential for study success?
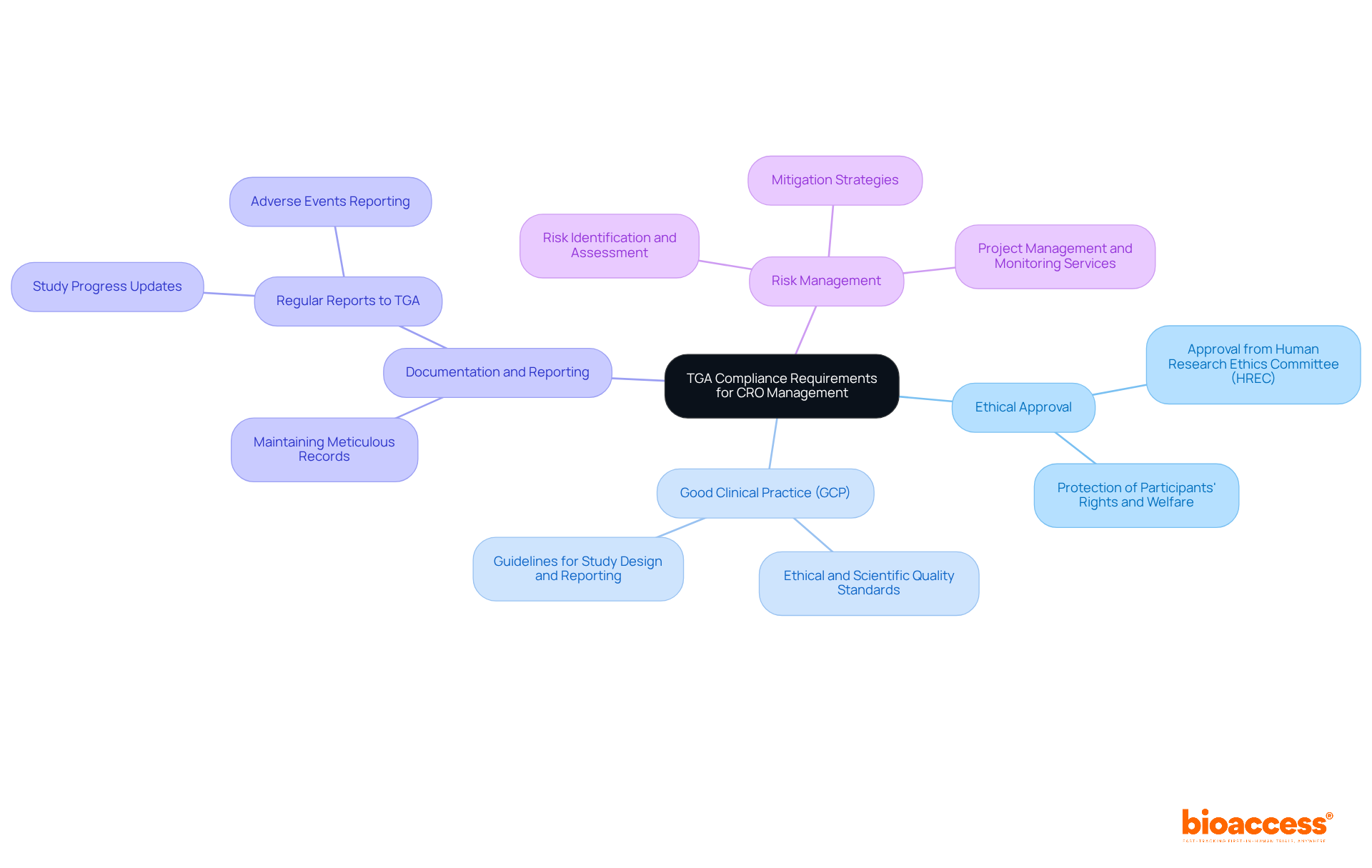
To effectively manage CROs in TGA-compliant studies, it is crucial to understand the Therapeutic Goods Administration (TGA) regulations. The TGA oversees the regulation of therapeutic products in Australia, ensuring that research studies adhere to safety, efficacy, and quality standards. Key compliance requirements include:
By grasping these regulatory requirements and leveraging the extensive clinical study management services provided by Bioaccess, CROs can excel in managing CROs in TGA-compliant studies to align their operations with TGA standards. This alignment not only enhances the credibility of clinical studies but also significantly boosts their chances of success.
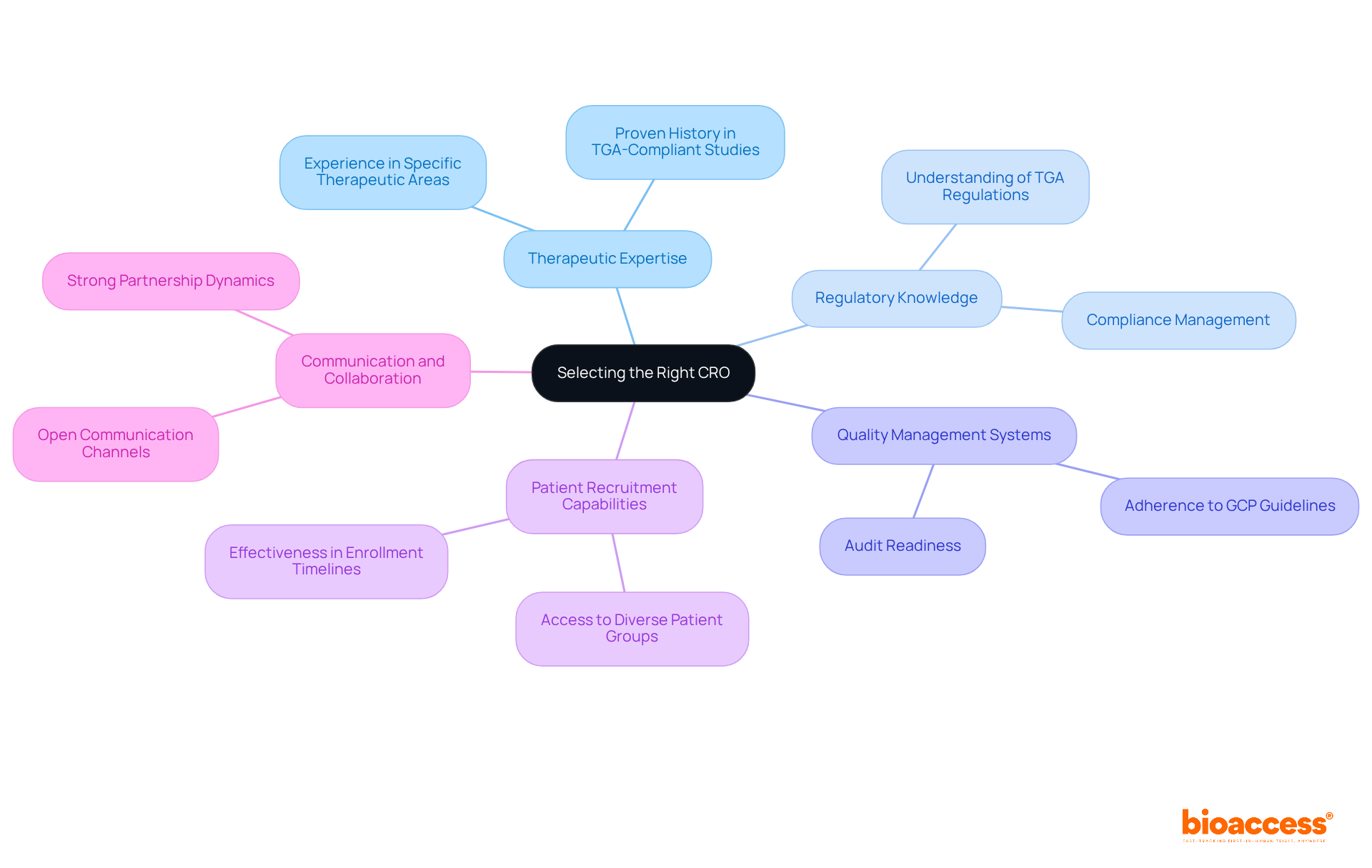
Selecting the right CRO is crucial for managing cros in tga-compliant studies and ensuring the success of your clinical research. A thorough evaluation of several essential criteria can help you make an informed decision:
By applying these criteria, sponsors can identify a CRO that aligns with their study objectives and regulatory requirements, which is essential for managing cros in tga-compliant studies, significantly increasing the likelihood of successful outcomes. Given that the overall success rate of medical studies is only 7.9%, selecting the right CRO is vital for managing the inherent risks. With bioaccess®'s expertise in managing Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies, we are well-positioned to support your clinical research needs.
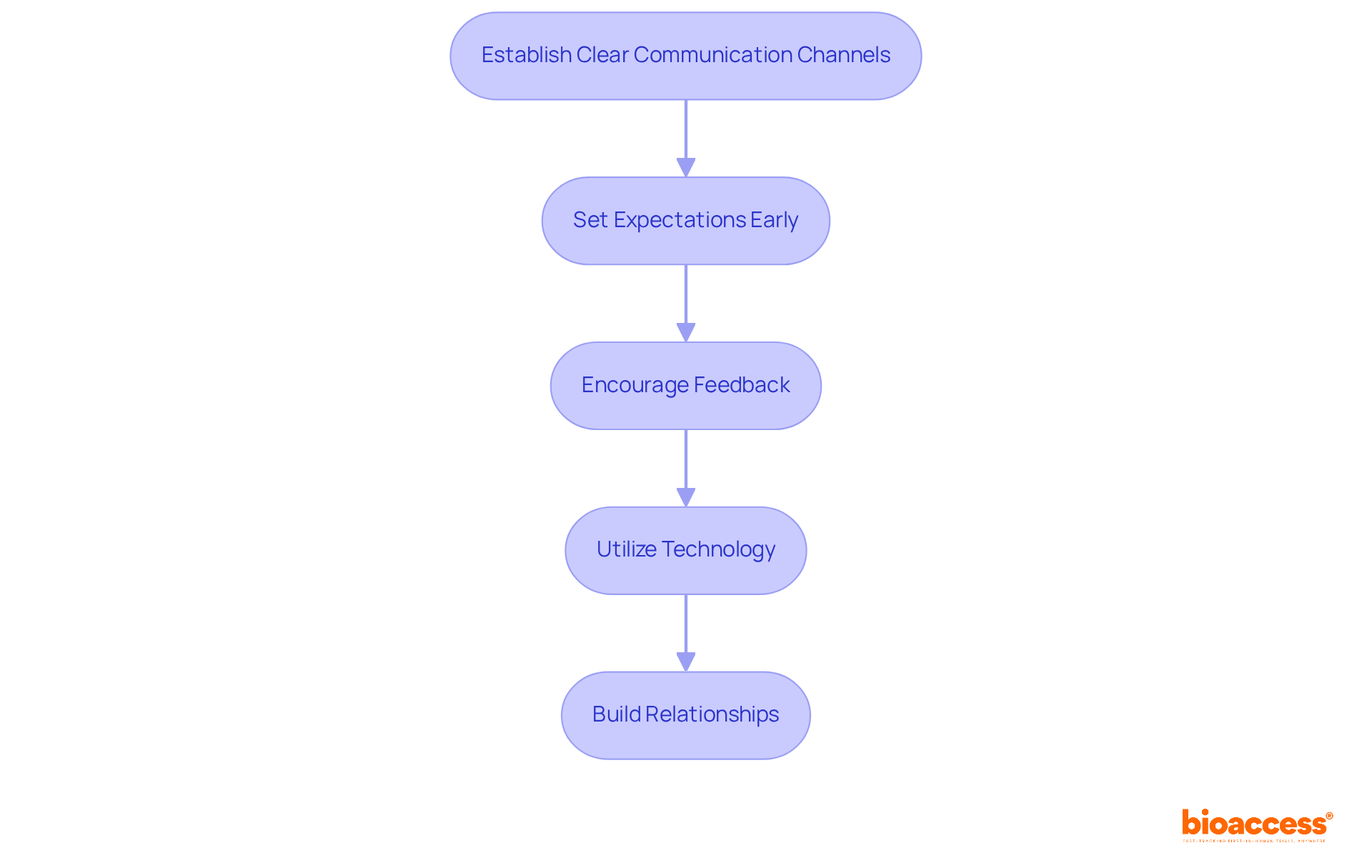
To foster effective communication and collaboration with CROs like bioaccess, consider the following strategies:
Establish Clear Communication Channels: Define preferred communication methods and schedule regular check-ins to discuss progress, challenges, and updates. This ensures alignment and keeps all parties informed.
Set Expectations Early: Clearly outline roles, responsibilities, and expectations from the outset. This proactive approach prevents confusion and establishes a collaborative tone for the partnership.
Encourage Feedback: Create an environment where feedback is welcomed and valued. Regularly solicit input from CRO teams to identify areas for improvement and enhance collaboration.
Utilize Technology: Leverage project management tools and communication platforms to facilitate real-time updates and document sharing. This streamlines workflows and enhances transparency.
Build Relationships: Invest time in developing personal relationships with CRO staff. Understanding their perspectives and challenges fosters a more cooperative working environment.
Applying these strategies can greatly improve collaboration with CROs, which is essential for managing cros in tga-compliant studies, resulting in more effective management and better outcomes.
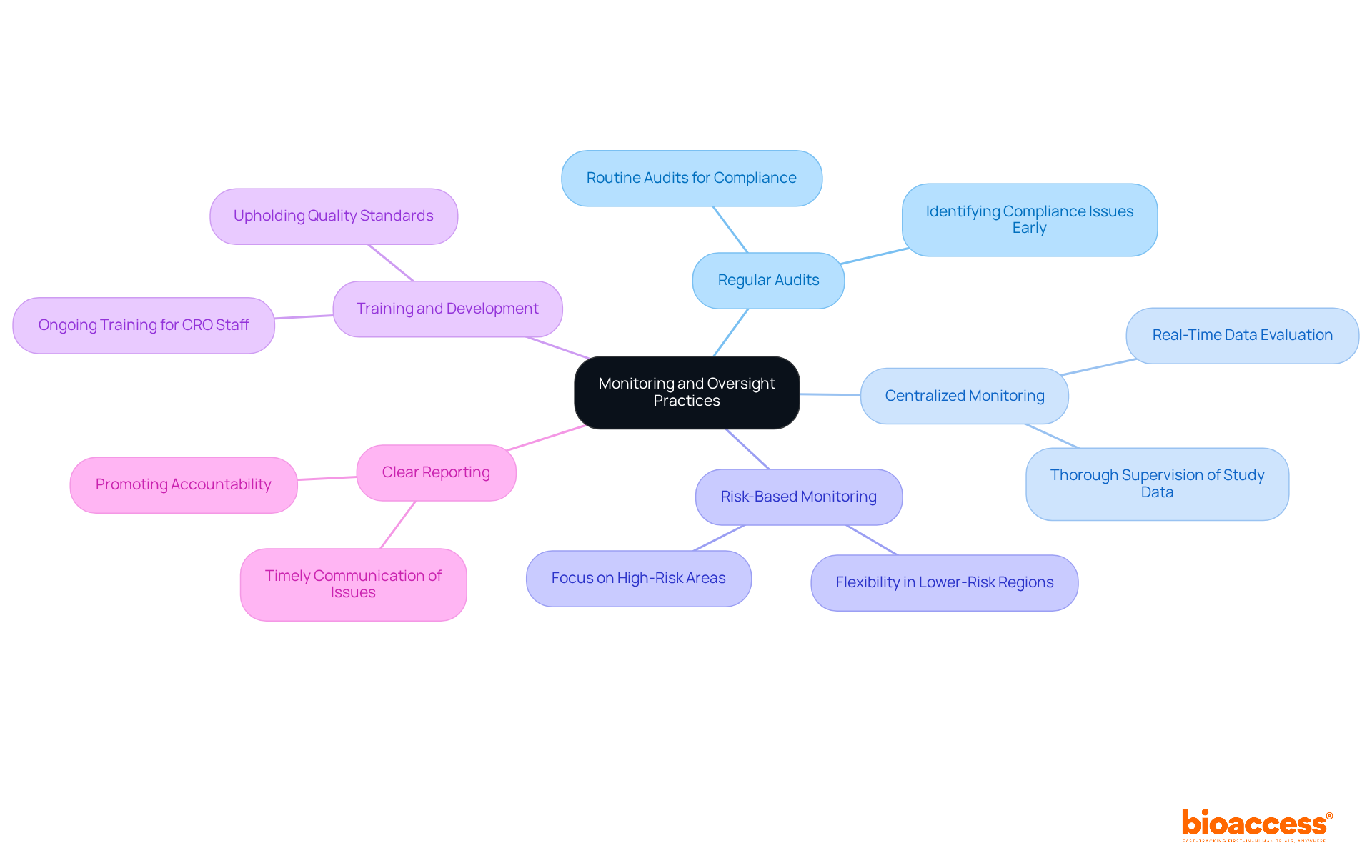
To ensure compliance and quality in clinical trials, implementing robust monitoring and oversight practices is essential:
Regular Audits: Routine audits of testing processes and documentation are crucial for compliance with Good Clinical Practice (GCP) and TGA regulations. This proactive strategy identifies potential compliance issues early, preventing escalation and ensuring the integrity of the process.
Centralized Monitoring: Employing centralized monitoring methods allows for thorough supervision of study data and site performance. This approach facilitates real-time data evaluation, enabling faster detection of discrepancies or problems that may arise during testing.
Risk-Based Monitoring: A risk-based monitoring strategy focuses resources on high-risk areas of the study, ensuring that critical elements receive thorough examination while allowing flexibility in lower-risk regions. This targeted approach enhances overall testing efficiency and safety.
Training and Development: Ongoing training for CRO staff on compliance requirements and best practices is vital. Well-trained staff are better equipped to uphold quality standards and effectively tackle challenges that may emerge during the testing process.
Clear Reporting: Establishing a clear reporting system fosters timely communication about study progress and any encountered issues. This transparency promotes accountability and ensures that all stakeholders remain informed throughout the process.
By implementing these practices, sponsors can significantly enhance the oversight of their CROs, which involves managing CROs in TGA-compliant studies while prioritizing patient safety.
Mastering the management of Contract Research Organizations (CROs) in TGA-compliant studies is not just important; it’s essential for the success and integrity of clinical research. Understanding the intricacies of TGA regulations and implementing best practices can significantly enhance operational efficiency and compliance, ultimately leading to more successful outcomes.
Key insights emphasize the necessity of:
Each of these elements plays a critical role in navigating the complexities of clinical trials while ensuring adherence to regulatory standards. Leveraging the expertise of organizations like Bioaccess can streamline these processes, from ethical approvals to patient recruitment and risk management.
In summary, the importance of managing CROs in TGA-compliant studies cannot be overstated. By applying the outlined best practices, stakeholders can improve their chances of success and contribute to the advancement of safe and effective therapeutic products. Embracing these strategies fosters a culture of compliance and collaboration, ultimately benefiting the broader landscape of clinical research.
What is the role of the Therapeutic Goods Administration (TGA) in clinical studies?
The TGA oversees the regulation of therapeutic products in Australia, ensuring that research studies adhere to safety, efficacy, and quality standards.
What is required for ethical approval in clinical studies?
All clinical studies must receive approval from a Human Research Ethics Committee (HREC) before commencing to protect the rights and welfare of participants.
What are Good Clinical Practice (GCP) guidelines?
GCP guidelines are ethical and scientific quality standards that CROs must follow for designing, conducting, recording, and reporting studies.
What documentation and reporting requirements do CROs have under TGA compliance?
CROs must maintain meticulous records and submit regular reports to the TGA, detailing study progress and any adverse events.
How does Bioaccess assist with documentation and reporting?
Bioaccess provides comprehensive reporting services that include study status updates and inventory management.
Why is risk management important in TGA-compliant studies?
Implementing a risk management plan is essential to identify, assess, and mitigate potential risks throughout the testing process.
What services does Bioaccess offer related to risk management?
Bioaccess offers project management and monitoring services to ensure that risks are effectively managed during clinical studies.
How can understanding TGA compliance requirements benefit CROs?
By grasping these regulatory requirements and leveraging Bioaccess's clinical study management services, CROs can enhance their credibility and improve the chances of success in TGA-compliant studies.